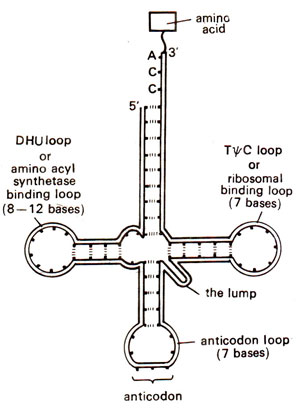
Fig. 31.3. Basic plan of the structure of tRNA.
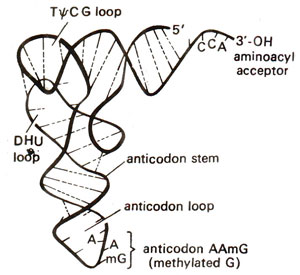
Fig. 31.4. A model for three dimensional structure (TDS) of tRNA showing L-shaped structure (as proposed by Kim, 1973).
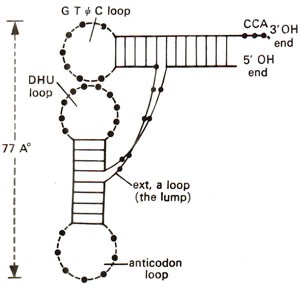
Fig. 31.5. A simplified diagrammatic representation' of L-shaped three dimensional structure (TDS) of tRNA as shown in Figure 31.4.
Two dimensional structure of tRNA (clover leaf model). Since amino acids can not directly recognize the base sequence in a mRNA, tRNA plays a very significant role in translation. It has to recognize
firstly, an amino acid through its corresponding amino acyl RNA synthetase enzyme,
secondly, the ribosome and
finally the base sequence in mRNA. From this view point, the detailed structure of these tRNA species assumes special significance. The basic plan of the structure af tRNAs takes up the pattern of a clover leaf and is given in Figure 31.3. Also, since only approximately 80 bases are present in one tRNA molecule, it was relatively easier to work out the detailed structure of several tRNA molecules.
Structures of different tRNAs for almost all amino acids are now available and fit the clover leaf model given in Figure 31.3. The first tRNA, for which in 1964 R.W.
Holley and his colleagues gave detailed structure, was alanyl tRNA (tRNA
ala) from yeast
(Holley received Nobel Prize for this work along with
Khorana and
Nirenberg in 1968). This knowledge was later used by Khorana and his colleagues for the synthesis of gene for this tRNA (
Genetic Engineering and Biotechnology 3. Isolation, Sequencing and Synthesis of Genes) On the basis of detailed structures of several species of tRNAs, some generalizations about their structure are listed below.
- All tRNAs contain a large number of unusual nucleosides e.g. pseudouridine (ψ or psi), inosine (I), dihydroxyuridine (DHU), etc. Methylation of normal bases is one of the commonest methods giving rise to these unusual bases.
- The ratios A : U and G : C are near unity which suggests the formation of DNA like double helical segments (secondary structure).
- In these double helical segments, G : C base pairs are more common than A : U as suggested by the ratio AU : GC = 0.7.
- All tRNA molecules have a tertiary structure, the details for which are now known, and Mg++ ion concentration is important for its stabilization. Therefore, although these are polynucleotides, their structure and function are much like those of enzymes.
- All tRNA molecules have guanine residue 'G' at the 5' terminal end and unpaired (single stranded) 'CCA' sequence at the 3' end. The amino acid is accepted at this 3' end only.
- The intermediate portion of a tRNA molecule seems to be invariably folded in a clover leaf pattern with three or more double helical regions, each having a loop. An 'anticodon' is usually present in the loop on the second helical region, and recognizes its corresponding 'codon' in rriRNA. However, the number of tRNAs is not necessarily as high as the number of codons with the result that one tRNA species can recognize several synonymous codons meant for the same amino acid (such codons meant for the same amino acid are known as synonyms). Conversely, a given codon may be recognized by more than one tRNA species. The other two side arms of tRNA carry loops which separately help, one in binding of amino acyl synthetase and the other in binding ribosome (Fig. 31.3).
Three dimensional structure of tRNA. In order to understand the structure- function relationship of tRNA, its three dimensional structure (TDS) should be known. With the availability of tRNA in crystalline form in 1968, TDS of tRNA could be studied through X-ray crystallography as was done for DNA double helix.
A. Klug from MRC laboratory, Cambridge, who was awarded 1982 Nobel Prize in Chemistry contributed much to the study of TDS of tRNAs. About a dozen models for TDS were initially proposed, but the latest and most acceptable model was proposed in 1973 by S.H.
Kim. He suggested that TDS of tRNA takes the shape of letter L with a thickness of 20 Å (Figs. 31.4, 31.5). This can be easily derived from two dimensional clover leaf model (Fig. 31.3). The extra arm which varies in different tRNA molecules can be extended without distorting TDS conformation. The CCA stem projects out and can take different orientations.

Fig. 31.3. Basic plan of the structure of tRNA.

Fig. 31.4. A model for three dimensional structure (TDS) of tRNA showing L-shaped structure (as proposed by Kim, 1973).

Fig. 31.5. A simplified diagrammatic representation' of L-shaped three dimensional structure (TDS) of tRNA as shown in Figure 31.4.
Extended anticodon and translational efficiency of t-RNA. In recent years it has been shown that the performance of anticodon, when isolated from tRNA, is weak and inaccurate. Il has also been shown that the performance of
triplet anticodon is enhanced if a matching sequence is present on the anticodon loop and on the stem on either side of anticodon triplet. For this purpose an
extended anticodon hypothesis has been formulated, according to which the structures of anticodon loop and that of the proximal anticodon stem are related to the sequence of anticodon. In other words, the anticodon is extended into the nearby sequence and consists of (i) two nucleotides at the 5' side of anticodon loop, (ii) three nucleotides of anticodon, (iii) two nucleotides at the 3' side of anticodon loop and (iv) five pairs of nucleotides in the anticodon stem which can be conveniently written by giving only the bases on 3' side of the stem.
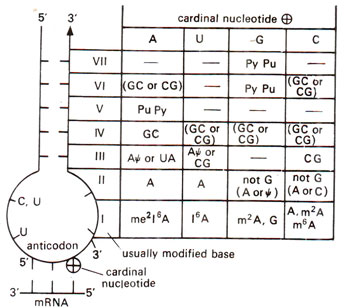
Fig. 31.6. General pattern of organisation of nucleotide sequence around the anticodon of tRNAs. On the left anticodon loop and its associated stem is shown. On the right the pattern of organization is shown, where at the top cardinal nucleotide at 3' position of anticodon is shown. In the body of the table the possible nucleotides in the anticodon loop stem, numbered I—VII are shown. A dash indicates, that any nucleotide may be present; Pu means purine (A or G), Py means pyrimidine (U or C); not G means A or U or C, (redrawn from Yarus, Science 218 : 1982).
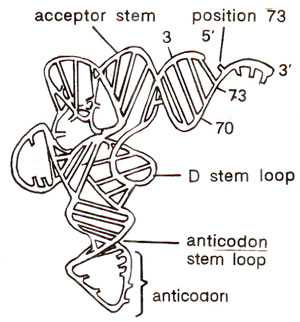
Fig. 31.7. Different recognition elements of a tRNA (modified from Saks et al., 1994; for details see text).
On the basis of the details of these 12 nucleotides for a number of tRNAs, a general scheme was prepared by
Michael Yarus in 1982, which is reproduced in Figure 31.6. In this scheme the 3' nucleotide of anticodon triplet (which will pair with 5' base of triplet codon) has been considered to be the most important and was called
cardinal nucleotide. It has been shown that there is some similarity in the sequences of extended anticodons with same or related
cardinal nucleotide. It is interesting to notice that if stem sequence and anticodon loop sequence at 3' end are known, anticodon triplet sequence can be deduced from Figure 31.6. It may also be noticed that 5' loop always has CU or ψU or UU suggesting that this sequence is strongly conserved. Similarly, 3' loop is strongly related with the function of tRNA. Even among tRNAs having same function with same anticodon triplet sequence, the efficiency may differ and may be attributed to differences in extended anticodon. This is illustrated with the help of a study of suppressor tRNAs which suppress amber (UAG codon) and ochre (UAA codon) mutants in
E. coli. These tRNAs result due to mutation in single base of anticodon. Whenever the suppressor tRNA originated due to change of
cardinal nucleotide at 3' end of anticodon, the suppression was found to be weak showing low efficiency, since it does not fit now in the pattern given in Figure 31.6. If the change takes place in any of the other two bases of anticodon, the suppression is strong and efficiency of translation is not affected. The details of these suppressor tRNAs in suggesting a role of extended anticodon in translational efficiency is discussed by Michael
Yarus (Science Vol. 218, 12 November, 1982).
Interaction of tRNA and amino acyl synthetase (second genetic code). In some
E. coli tRNAs, it has been shown that binding of tRNAs to amino acyl synthetase enzyme takes place by formation of a covalent bond between uridine 8 of a tRNA (uridine is present at position 8 of the nucleotide chain of all cytoplasmic tRNAs)and its corresponding aminoacyl synthetase enzyme. It is suggested that this covalent bond formation is essential for the activity of amino acyl synthetase enzyme. Uridine 8 of tRNA is exposed outside even in a three dimensional structure of tRNA, so that the covalent bond formation can be easily achieved.
One of the major problems in tne study of tRNA structure and function has been to find out the elements of tRNA that permit an aminoacyl-tRNA synthetase to recognize and aminoacylate its corresponding tRNA, avoiding tRNAs for all other amino acids. Although there are no general rules for all tRNAs, the following recognition elements in tRNAs have been identified : (i) the anticodon, (ii) the acceptor stem, (iii) position 73 (towards 3' end), (iv) the variable loop and (v) variable pocket. In tRNAs from most isoaccepting groups, there are recognition elements in at least two locations, most common of them being anticodon, and acceptor stem or the base at position 73 (or both). In 17 of the 20 isoaccepting groups in
E. coli at least one of the three nucleosides in the anticodon contributes to recognition. (An isoaccepting group of tRNAs means that all these tRNAs accept same amino acid). These recognition elements are shown in Figure 31.7 (for more details consult Saks
et al., 1994).

Fig. 31.6. General pattern of organisation of nucleotide sequence around the anticodon of tRNAs. On the left anticodon loop and its associated stem is shown. On the right the pattern of organization is shown, where at the top cardinal nucleotide at 3' position of anticodon is shown. In the body of the table the possible nucleotides in the anticodon loop stem, numbered I—VII are shown. A dash indicates, that any nucleotide may be present; Pu means purine (A or G), Py means pyrimidine (U or C); not G means A or U or C, (redrawn from Yarus, Science 218 : 1982).

Fig. 31.7. Different recognition elements of a tRNA (modified from Saks et al., 1994; for details see text).
The problem of recognition of tRNAs belonging to different isoaccepting groups by their corresponding aminoacyl-tRNA synthetases is often described as the Second Genetic Code (although this 'phrase' has been considered misleading by some). In recent years, availability of new technologies helped in the study of this problem to give a reasonably complete picture of how tRNAs are recognised. Some of these new technologies include the following : (i) Computer search for distinct or unique nucleotides in individual tRNAs; (ii) aminoacylation and binding ability of tRNAs that have altered domains introduced enzymatically or by chemical synthesis or by site directed mutagenesis; (iii) amino acid sequencing of proteins synthesized using mutant tRNAs; (iv) x-ray crystallography and NMR analyses of tRNA-synthetase complexes (see next para).
The aminoacyl-tRNA synthetases form a family of proteins characterized by their high degree of structural diversity and limited sequence homology. Crystal structures of several of these enzymes from
E. coli have been examined by X-ray crystallography. Some of these enzymes form active dimers in solution, but other exist as monomers (glutaminyl-tRNA synthetase). Crystal structure of a complex between glutaminyl-tRNA synthetase and tRNA
Gln was also examined in 1990. It was shown that the enzyme has an elongated ellipsoidal shape divided in four domains, of which the most important is a five-stranded β sheet folded in a specific manner
(Rossman fold) to perform its dual function of recognizing tRNA
Gln and catalyzing the linkage of tRNA
Gln with amino acid 'glutamine'. There are other synthetases (Ser-tRNA synthetase), where such a Rossman fold is missing, so that the synthetases may be divided in two or more groups.
Glutaminyl-tRNA synthetase structure suggests that the tip of the long arm of L shaped tRNA fits into deep little pocket in the protein, but the interaction mainly involves two bases of anticodon, thus helping to explain how one synthetase recognizes more than one tRNAs for the same amino acid. Another interaction occurs at the tip of the short arm of L, which is inserted into a
gaping cavern (hollow cave) in the enzyme. This cavernous active site catalyzes the formation of link between amino acid and tRNA. ATP molecule is bound at the lower surface of cavern to supply energy during formation of linkage. More information will be forthcoming about these interactions during 1990s.














