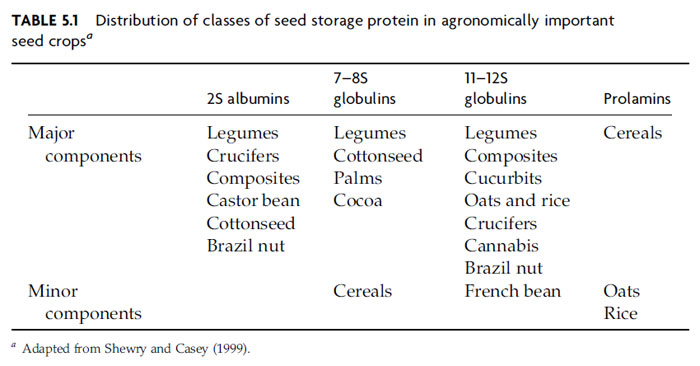
Characterization of Seed Storage Proteins
A modern classification system for seed proteins separates them into storage
proteins, structural and metabolic proteins, and protective proteins, with certain
proteins belonging to more than one of these classes (Shewry and Casey, 1999).
Based on the knowledge of their molecular structure, the major groups of seed
storage proteins are now classified as prolamins, 2S albumins, 7–8S globulins, and
11–12S globulins, where S refers to the sedimentation coefficient (Shewry and
Casey, 1999). The distribution of these proteins in economically important crops is
shown in Table 5.1. In general, globulins and albumins are the major components
in dicotyledonous species, whereas prolamins predominate in most cultivated
cereals.
 |
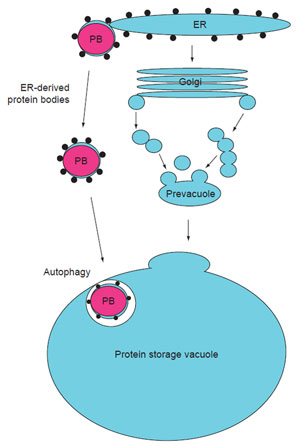 |
| FIGURE 5.1 Diagram illustrating the ontogeny of PBs and protein storage vacuoles (PSVs). PBs form through the aggregation of storage proteins within the ER or PSVs. After formation, PBs can either remain attached to the ER or bud off and form separate organelles, that is PSVs. PBs can accumulate in the cytoplasm or become sequestered into PSVs by autophagy. PSVs are formed as the consequence of ER-synthesized storage proteins progressing through the endomembrane secretory system to specialized vacuoles (PSVs) for accumulation. Reprinted from Herman and Larkins (1999) with permission from the ASPB. |
Seed storage proteins are synthesized on rough endoplasmic reticulum (ER)
membranes. They can be retained in the ER as localized protein accretions (protein
bodies or PBs) or they can be transported, often via the Golgi complex, to
specialized protein storage vacuoles (PSVs). PBs become deposited in PSVs either
directly through autophagy or through the endomembrane secretory system.
These pathways are illustrated in Fig. 5.1.
Prolamins
Prolamins were the first group of storage proteins to be widely studied. They
account for about half of the grain nitrogen in most cereals, although as with other
types of storage proteins, their levels vary considerably, depending on nitrogen and sulfur availability (Shewry
et al., 1983; Tabe
et al., 2002). Prolamins are
synthesized on rough ER membranes, and they can form accretions (PBs) directly
in the ER or be transported into specialized PSVs (Fig. 5.1) (Herman and Larkins,
1999). In corn and wheat, prolamins account for about 60–70% of the endosperm
protein, whereas in oats and rice they account for less than 10% of the protein
(Shewry and Tatham, 1999).
Prolamins have been classified according to size and sulfur content, but no
standard nomenclature exists for their classification between species. Prolamins
are typically very rich in proline and glutamine, and are deficient, if not devoid,
of several essential amino acids, including lysine, tryptophan, tyrosine, and threonine.
As a result, monogastric animals receiving diets in which cereals are the
primary protein source often develop protein deficiency disorders (Bhan
et al.,
2003). In humans, such a deficiency is called kwashiorkor that, in addition to
retarding growth and development, causes immunologic impairment and thus
susceptibility to life-threatening infections (Scrimshaw, 2003). In some cereals,
mutations have been found that reduce prolamin synthesis while increasing the
proportion of more nutritional types of proteins (Habben and Larkins, 1995;
Nelson, 2001). However, such mutants are generally associated with deleterious
phenotypes, and for the most part have not been commercially developed. The
fact that all classes of prolamin genes encode proteins deficient in essential amino
acids means that such nutritional deficiencies are not amenable to correction by
conventional plant breeding. Consequently, molecular biologists have sought to
improve cereal protein quality by genetic engineering of genes encoding proteins
with high levels of essential amino acids. Since prolamins also affect the functional
characteristics of cereal flours, such as the bread-making quality of wheat (Shewry
and Halford, 2002; Shewry
et al., 2003a) and the digestibility of the grain (Oria
et al., 2000), there is also interest in increasing or decreasing the synthesis of
particular types of prolamin proteins.
Globulins
Globulins are present to some extent in all seeds of all plants but they are the main
storage proteins in most dicots and certain monocots, such as oats and rice
(Table 5.1). The major storage globulins comprise the 11–12S and 7S groups and
are often called legumins and vicilins, the common names given to the 11S and 7S
proteins in peas. However, the 11–12S and 7S proteins typically have common
names in each species (Casey, 1999). The 7S globulins exist as trimeric structures
with subunit sizes of 50–70 kDa (Lawrence
et al., 1994), and the 11–12S globulins are hexamers with subunit sizes 60–80 kDa (Adachi
et al., 2003). Their size
variation is due to differences in primary structure as well as posttranslational
modifications. During synthesis, subunits of the proteins pass through the ER and
(in some cases) the Golgi body (Fig. 5.1). They undergo partial assembly in the
ER and are finally deposited in PSVs derived from the large central vacuole
(Herman and Larkins, 1999; Kermode and Bewley, 1999). Dicot seeds, especially
legumes, are rich sources of protein but the low levels of methionine (an essential
amino acid) and cysteine in their storage globulins limit their nutritional value.
Consequently, increasing the level of these sulfur-containing amino acids is a
major goal for their improvement through biotechnology).
Albumins
Albumins were first defined as a separate group of seed proteins on the basis of
their water solubility (Osborne, 1924), but it was not until the 1980s that sucrose
density gradient sedimentation was used to definitively identify storage proteins
of this type in seeds from a diverse range of species (Shewry and Pandya, 1999;
Youle and Huang, 1981). Albumins have sedimentation coefficients of 2S, and
though they exhibit substantial sequence and structural polymorphism between
species, some amino acid conservation exists. Albumins typically exist in heterodimeric
forms, comprising 30–40 and 60–90 amino acid subunits, which are
derived from a precursor protein. Assembly occurs in the lumen of the ER, after
which the proteins are delivered to PSVs for final proteolytic processing and
deposition (Fig. 5.1). There has been considerable interest in 2S albumins because
of their high cysteine and methionine contents (Youle and Huang, 1981).

