Immobilization of Enzymes
Thus, immobilization is "the imprisonment of an enzyme in a distinct phase that allows exchange with, but is separated from the bulk phase in which the substrate, effector or inhibitor molecules are dispersed and monitored" (Trevan, 1980). Imprisonment refers to arresting the enzyme by certain means where polymer matrix is formed. The first commercial application of immobilized enzyme technology was realized in 1969 in Japan with the use of Aspergillus oryzae amino acylase for the industrial production of L-amino acids. Consequently, pilot plant processes were introduced for 6-amino penicillanic acid (6 APA) production from penicillin G and for glucose to fructose conversion by immobilized glucose isomerase.
The advantages of using immobilized enzymes are : (i) reuse (ii) continuous use (iii) less labor intensive (iv) saving in capital cost (v) minimum reaction time (vi) less chance of contamination in products, (vii) more stability (viii) improved process control and (ix) high enzyme : substrate ratio.
The first immobilized enzymes to be scaled up to pilot plant level and industrial manufacture were immobilized amino acid acylase, panicillin G-acylase and glucose isomerase. Some other industrially important enzymes are aspartase, esterase and nitrilase.
Methods of Enzyme Immobilization
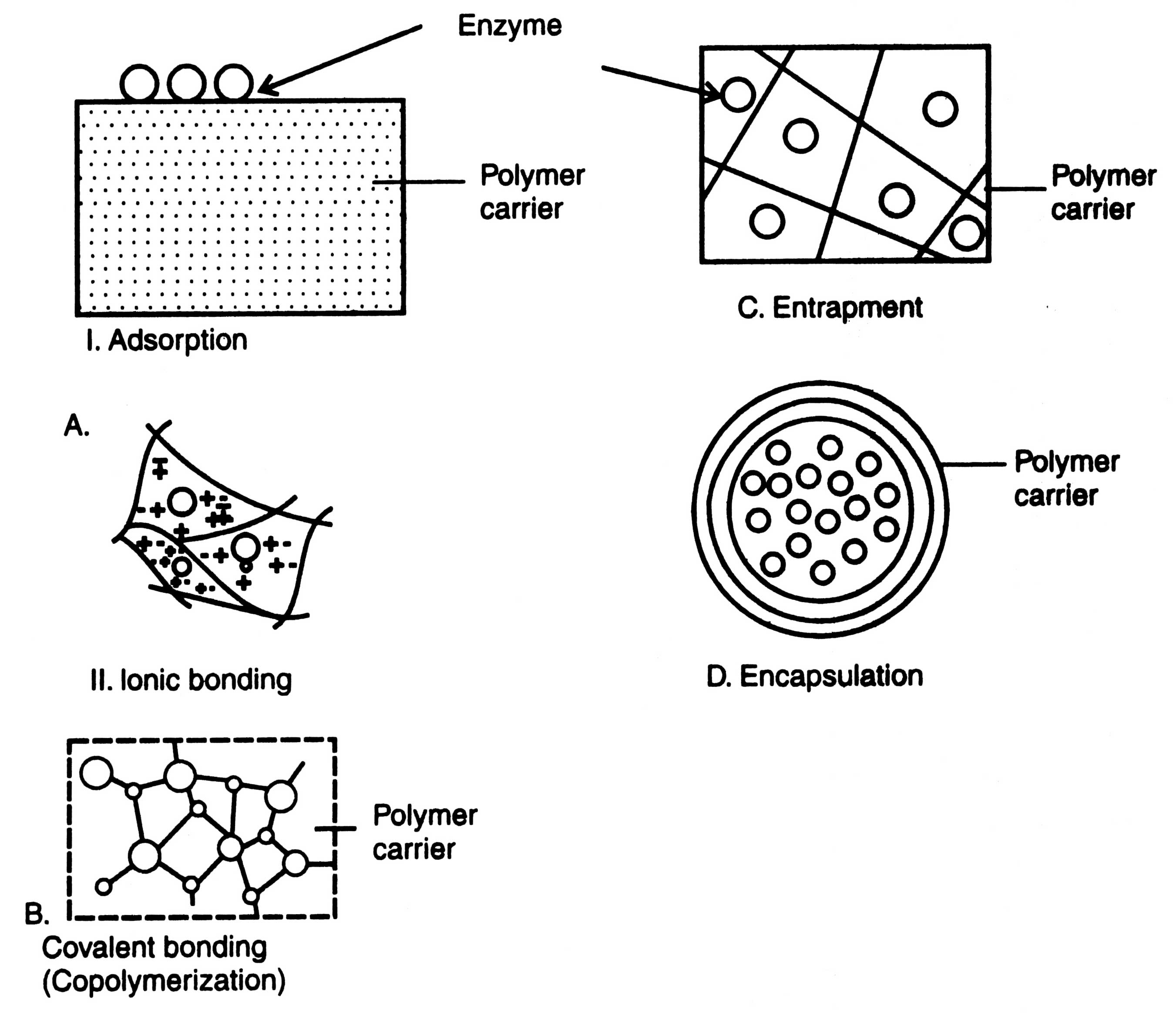
There are five different techniques of immobilizing enzymes : (i) adsorption, (ii) covalent bonding, (iii) entrapment, (iv) copolymerization or cross-linking, and (v) encapsulation (Fig. 17.1). For the purpose of immobilization of enzymes carriers i.e. the support materials such as matrix system, a membrane or a solid surface are used.
An enzyme may be immobilized by bonding to either external or internal surface of a carrier or support such as mineral support (aluminium oxide, clay), organic support (starch), modified sapharose and ion exchange resins. Bonds of low energy are involved e.g. ionic interactions, hydrogen bonds, van der Waals forces, etc. If the enzyme is immobilized externally, the carrier particle size must be very small in order to achieve a appreciable surface of bonding. These particles may have diameter ranging from 500 A to about 1 mm. Due to immobilization of enzymes on external surface, no pore diffusion limitations are encountered.
In addition, the enzyme immobilized on an internal surface is protected from abrasion, inhibitory bulk solutions and microbial attack, and a more stable and active enzyme system may be achieved. Moreover, in internal pore immobilization the pore diameters of carriers may be optimized for internal surface immobilization (Fig. 17.1 A ).
Covalent bonding
Covalent bond is formed between the chemical groups of enzyme and chemical groups on surface of carrier. Covalent bonding is thus utilized under a broad range of pH, ionic strength and other variable conditions. Immobilization steps are attachment of coupling agent followed by an activation process, or attachment of a functional group and finally attachment of the enzyme (Fig. 17.1B).
Different types of carriers are used in immobilization such as carbohydrates proteins and amine-bearing carriers, inorganic carriers, etc. Covalent attachment may be directed to a specific group (e.g. amine, hydroxyl, tyrosyl, etc.) on the surface of the enzyme. Hydroxyl and amino groups are the main groups of the enzymes with which it forms bonds, whereas sulphydryl group least involved.
Different methods of covalent bonding are : (i) diazoation (bonding between the amino group of the support e.g. aminobenzyle cellulose, aminosilanised porous glass, aminoderivatives and a tyrosyl or histidyl group of the enzyme), (ii) formation of peptide bond (bond formation between the amino or carboxyl group of the support and amino or carboxy group of the enzyme), (iii) group activation (use of cyanogen bromide to a support containing glycol group i.e. cellulose, syphadex, sepharose, etc), and (iv) poly functional reagents (use of a bifunctional or multifunctional reagent e.g. glutaraldehyde which forms bonding between the amino group of the support and amino group of the enzyme).
The major problem with covalent bonding is that the enzyme may be inactivated by bringing about changes in conformation when undergoes reactions at active sites. However, this problem can be overcome through immobilization in the presence of enzyme's substrate or a competitive inhibitors or protease. The most common activated polymers are celluloses or polyacrylamides onto which diazo, carbodimide or azide groups have been incorporated.
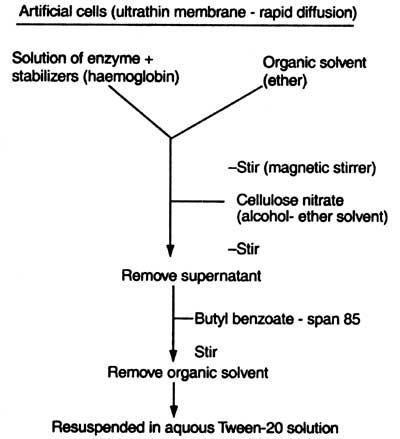
Fig. 17.2. Enzyme encapsulation (after Chent, 1977)

Fig. 17.3. A SEM of immobilized cells of yeast (Saccharomyces cerevaiae) with in a solid phase matrix (with permission from Dr. A.J. Knights, Univ. Wales, Swansea).
Enzymes can be physically entrapped inside a matrix (support) of a water soluble polymer such as polyacrylamide type gels and naturally derived gels e.g. cellulose triacetate, agar, gelatin, carrageenan, alginate, etc. (Fig. 17.1C). The form and nature of matrix vary. Pore size of matrix should be adjusted to prevent the loss of enzyme from the matrix due to excessive diffusion. There is possibility of leakage of low molecular weight enzymes from the gel. Agar and carrageenan have large pore sizes (< 10m).
There are several methods for enzyme entrapment: (i) inclusion in gels (enzyme entrapped in gels), (ii) inclusion in fibers (enzyme entrapped in fiber format), and (iii) inclusion in microcapsules (enzymes entrapped in microcapsules formed monomer mixtures such as polyamine and polybasic chloride, polyphenol and polyisocyanate). The entrapment of enzymes has been widely used for sensing application, but not much success has been achieved with industrial process.
Cross-linking or Co-polymerization
Cross-linking is characterized by covalent bonding between the various molecules of an enzyme via a polyfunctional reagent such as glutaraldehyde, diazonium salt, hexamethylene disocyanate, and N-N' ethylene bismaleimide. The demerit of using polyfunctional reagents is that they can denature the enzyme. This technique is cheap and simple but not often used with pure proteins because it produces very little of immobilized enzyme that has very high intrinsic activity. It is widely used in commercial preparation.
Encapsulation is the enclosing of a droplet of solution-of enzyme in a semipermeable membrane capsule. The capsule is made up of cellulose nitrate and nylon. The method of encapsulation is cheap and simple but its effectiveness largely depends on the stability of enzyme although the catalyst is very effectively retained within the capsule. This technique is restricted to medical sciences only (Fig. 17.1D).
In this method a large quantity of enzyme is immobilized but the biggest disadvantage is that only small substrate molecule is utilized with the intact membrane. Chent (1977) has given the method of enzyme encapsulation (Fig. 17.2).
In the field of enzyme technology, immobilization of whole cells is now a well developed method. Successful performance of several industrial plants has been demonstrated. In cell immobilization technology the main important feature is that enzymes are active and stable for a long period of time. It keeps within the cellular domain together with all cell constituents whether the cells are dead or viable but in resting state.
The methods of whole cell immobilization are same as described for enzyme immobilization i.e. adsorption, covalent bonding, cell to cell cross-linking, encapsulation, and entrapment in polymeric network. Since a long time adsorption of cells to preformed carrier has been done, for example use of woodchips as carrier for Acetobacter has been in practice for vinegar production since 1823. Preformed carrier of ones choice is used (Table 17.2). Cell attachment to the surface of preformed carrier is done by covalent bonding.
Table 17.2. Immobilization of whole cells by different methods.
| Support material | Cells | Reaction |
| A. Adsorption | ||
| Gelatin | Lactobacilli | Lactose/lactic acid |
| Porous glass | Saccharomyces carlsbergensis | Glucose/ethanol |
| Cotton fibers | Zymomonas mobilis | Glucose/ethanol |
| Vermiculite | Z. mobilis | Glucose/ethanol |
| DEAE-cellulose | Nocardia erythropolis | Steroid conversion |
| B. Covalent Bonding | ||
| Cellulose + cyanuric chloride | S.cerevisiae | Glucose/ethanol |
| Ti (IV) oxide, etc. | Acetobacter sp. | Wort/vinegar |
| Carboxymethylcellulose + carbodiimide | Bacillus subtilis | L-histidine/uronic acid |
| C. Crosslinking of cell-to-cell | ||
| Diazotized diamines | Streptomyces | Glucose/fructose |
| Glutaraldehyde | E.coli | Fumaric acid/L-aspartic acid |
| Flocculation by chitosan | Lactobacillus brevis | Glucose/fructose |
| D. Entrapment | ||
| Al alginate | Candida tropicalis | Phenol degradation |
| Ca alginate | S. cerevisiae | Glucose/ethanol |
| Mg pectinate | Fungi | Glucose/fructose |
| K-carrageenan | E.coli | Fumaric acid/L-spartic acid |
| Chitosan alginate | S. cerevisiae | Glucose/ethanol |
| E. Encapsulation | ||
| Cellulose acetate | Comamonas sp. | 7-ACA production |
| Ethylcelhilose | Streptomyces sp. | Glucose/fructose |
| Polyester | Streptomyces sp. | Glucose/fructose |
| Alginate-polylysine | Pancreas cells | - |
| Alginate-polylysine | Hybridoma cells | Monoclonal antibodies |