The Aspartate Family Pathway is Regulated by Several Feedback Inhibition Loops
In plants, as in many bacterial species, lysine, threonine, methionine, and isoleucine
are synthesized from aspartate through several different branches of the
aspartate family pathway (Fig. 3.2). While one branch of this pathway leads
to lysine biosynthesis, a second branch leads to threonine, isoleucine, and methionine
biosynthesis. Methionine and threonine biosyntheses diverge into two
subbranches and compete for
O-phosphohomoserine as an intermediate (Fig. 3.2).
The entire aspartate family pathway, except for the last step of methionine
synthesis (methionine synthase), occurs in the plastid. Although methionine is
often considered part of the aspartate family pathway, its biosynthesis is subject
to a special regulatory pattern, apparently due to its multiple functions in plants.
Therefore, we will discuss the regulation of methionine biosynthesis in a separate
section.
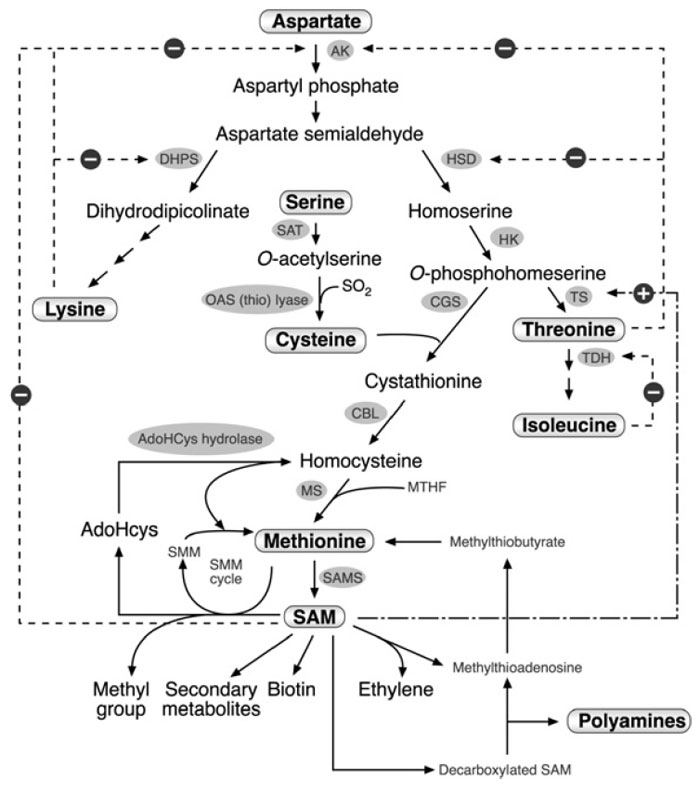 |
| FIGURE 3.2 Schematic diagram of the metabolic network containing the aspartate family
pathway, methionine metabolism, and last two steps in the cysteine biosynthesis. Only some of
the enzymes and metabolites are specified. Abbreviations: AK, aspartate kinase; DHPS,
dihydrodipicolinate synthase; HSD, homoserine dehydrogenase; HK, homoserine kinase; TS,
threonine synthase; TDH, threonine dehydratase; SAT, serine acetyl transferase; OAS (thio)
lyase; O-acetyl serine (thio) lyase; CGS, cystathionine γ-synthase; CBL, cystathionine β-lyase;
MS, methionine synthase, SAM, S-adenosyl methionine; SAMS, S-adenosyl methionine
synthase; AdoHcys, adenosylhomocysteine; SMM, S-methyl methionine; MTHF, methyltetrahydrofolate.
Dashed arrows with a ‘‘minus’’ sign represent feedback inhibition loops of key enzymes
in the network. The dashed and dotted arrow with the ‘‘plus’’ sign represents the stimulation of TS
activity by SAM. |
Biochemical studies showed that the aspartate family pathway is regulated by
several feedback inhibition loops (see Galili, 1995 for details; Fig. 3.2). Aspartate
kinase (AK) consists of several isozymes, five in
Arabidopsis, which are feedback
inhibited either by lysine or threonine. These include monofunctional polypeptides
containing either the lysine-sensitive AK activity, or bifunctional AK/HSD
enzymes containing both the threonine-sensitive AK and homoserine DH (HSD)
isozymes linked on a single polypeptide (see Galili, 1995). Lysine also feedback
inhibits the activity of dihydrodipicolinate synthase (DHPS), the first enzyme committed to its own synthesis, while threonine partially inhibits the activity of
HSD, the first enzyme committed to the synthesis of threonine and methionine.
Although both the monofunctional AK and DHPS activities are feedback
inhibited by lysine, DHPS is the major limiting enzyme for lysine biosynthesis,
while AK is a major limiting enzyme in the second branch of the aspartate family
pathway leading to threonine, isoleucine, and methionine biosynthesis.
This has
been concluded based on the analysis of plant mutants as well as transgenic plants
expressing recombinant feedback insensitive DHPS and AK enzymes derived
from either bacteria or plant sources (Galili, 1995, 2002; Galili and Hofgen, 2002;
Galili
et al., 1995; Jacobs
et al., 1987; 2001). The results of these functional studies
had been expected since the
in vitro activities of plant DHPS enzymes are much
more sensitive to lysine inhibition than those of the lysine-sensitive AK enzymes
(see Galili, 1995 for review).
Do the lysine and threonine branches compete for the common substrate
aspartate semialdehyde (Fig. 3.2)? Lysine overproduction in plants expressing a
feedback-insensitive DHPS is also generally associated with reduced levels of
threonine (Galili, 1995, 2002). Moreover, when the feedback-insensitive DHPS
and AK were combined into the same plant, lysine levels far exceeded those of
threonine levels (Ben Tzvi-Tzchori
et al., 1996; Frankard
et al., 1992; Shaul and
Galili, 1993). This suggests that apart from regulation by the feedback inhibition
loops of AK and DHPS, the lysine branch exerts a more powerful drain on
metabolic flux than the threonine branch.





