Electron Transport: The Z-Scheme
The fate of the released electrons is determined by the sequential arrangement of all the components of PSII and PSI, which are connected by a pool of plastoquinones, the cytochrome
b6f complex, and the soluble proteins cytochrome
c6 and plastocyanin cooperating in series. The electrons from PSII are finally transferred to the stromal side of PSI and used to reduce NADP
+ to NADPH, which is catalyzed by ferredoxin-NADP
+ oxidoreductase (FNR). In this process, water acts as electron donor to the oxidized P
680 in PSII, and dioxygen (O
2) evolves as a by-product.
Photosystem II uses light energy to drive two chemical reactions: the oxidation of water and the reduction of plastoquinone. Photochemistry in PSII is initiated by charge separation between P
680 and pheophytin, creating the redox couple P
+680/Pheo
-. The primary charge separation reaction takes only a few picoseconds. Subsequent electron transfer steps prevent the separated charges from recombining by transferring the electron from pheophytin to a plastoquinone molecule
within 200 ps.
The electron on Q
A-is then transferred to Q
B-site. As already stated, plastoquinone at the Q
B-site differs from plastoquinone at the Q
A-site in that it works as a two-electron acceptor and becomes fully reduced and protonated after two photochemical turnovers of the reaction center. The full reduction of plastoquinone at the Q
B- site requires the addition of two electrons and two protons. The reduced plastoquinone (plastoquinol, Q
BH
2) then unbinds from the reaction center and diffuses in the hydrophobic core of the membrane, after which an oxidized plastoquinone molecule finds its way to the Q
B-binding site and the process is repeated. Because the Q
B-site is near the outer aqueous phase, the protons added to plastoquinone during its reduction are taken from the outside of the membrane. Electrons are passed from Q
BH
2 to a membrane-bound cytochrome
b6f, concomitant with the release of two rotons to the luminal side of the membrane.
The cytochrome
b6f then transfers one electron to a mobile carrier in the thylakoid lumen, either plastocyanin or cytochrome
c6. This mobile carrier serves an electron donor to PSI reaction center, the P
700. Upon photon absorption by PSI a charge separation occurs with the electron
fed into a bound chain of redox sites; a chlorophyll a (A0), a quinone acceptor (A1) and then a bound Fe–S cluster, and then two Fe–S cluster in ferredoxin, a soluble mobile carrier on the stromal side. Two ferredoxin molecules can reduce NADP
+ to NADPH, via the flavoprotein ferredoxin-NADP
+ oxidoreductase. NADPH is used as redox currency for many biosynthesis reactions such as CO
2 fixation. The energy conserved in a mole of NADPH is about 52.5 kcal/mol, whereas in an ATP hole is 7.3 kcal/mol.
The photochemical reaction triggered by P
700 is a redox process. In its ground state, P
700 has a redox potential of 0.45 eV and can take up an electron from a suitable donor, hence it can perform an oxidizing action. In its excited state it possesses a redox potential of more than -1.0 eV and can perform a reducing action donating an electron to an acceptor, and becoming P
700+. The couple P
700/P
700+ is thus a light-dependent redox enzyme and possesses the capability to reduce the most electronnegative redox system of the chloroplast, the ferredoxin-NADP
+ oxidoreductase (redox potential = -0.42 eV). In contrast, P
700 in its ground state (redox potential = 0.45 eV) is not
able to oxidize, that is, to take electrons from water that has a higher redox potential (0.82 eV). The transfer of electrons from water is driven by the P
680 at PSII, which in its ground state has a sufficiently positive redox potential (1.22 eV) to oxidize water. On its excited state, P
680 at PSII reaches a redox potential of about –0.60 eV that is enough to donate electron to a plastoquinone (redox potential = 0 eV) and then via cytochrome
b6f complex to P
700+ at PSI so that it can return to P
700 and be excited once again. This reaction pathway is called the “Z-scheme of photosynthesis,” because the redox diagram from P
680 to P
700 looks like a big “Z” (Figure 3.4).
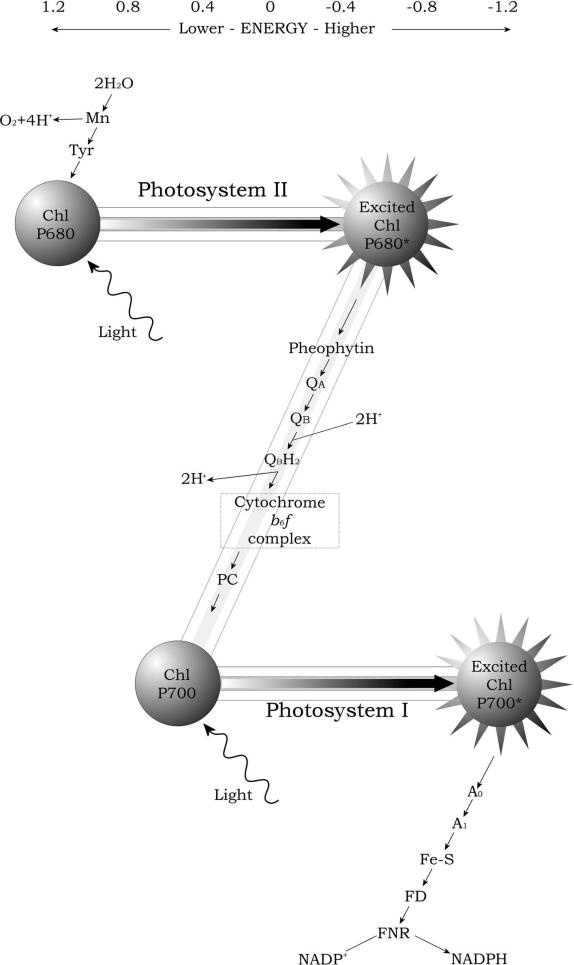
FIGURE 3.4 Schematic drawing of the Z-scheme of photosynthetic electron transport, with the positions of the participants on the oxido-reduction scale.
From this scheme it is evident that only approximately one third of the energy absorbed by the two primary electron donors P
680 and P
700 is turned into chemical form. A 680 nm photon has an energy of 1.82 eV, a 700 nm photon has an energy of 1.77 eV (total = 3.59 eV) that is three times more than sufficient to change the potential of an electron by 1.24 eV, from the redox potential of the water (0.82 eV) to that of ferredoxin-NADP
+ oxidoreductase (-0.42 eV).
It is worthwhile to emphasize that any photon that is absorbed by any chlorophyll molecule is energetically equivalent to a red photon because the extra energy of an absorbed photon of shorter wavelength (<680 nm) is lost during the quick fall to the red energy level that represents the lowest excited level.





