Bioremediation of Xenobiotics
The characters of pesticide degradation of microorganisms are located on plasmids and transposons, and are grouped in clusters on chromosome. Understanding of the characters provides clues to the evolution of degradative pathways and makes the task of gene manipulation easier to construct the genetically engineered microbes capable of degrading the pollutants.
Microbial Degradation of Xenobiotics
Biodegradation of pesticides occurs by aerobic soil microbes. Pesticides are of wide varieties of chemicals e.g. chlorophenoxyalkyl caboxylic acid, substituted ureas, nitrophenols, tri-azines, phenyl carbamates, orga-nochlorines, organophosphates, etc. Duration of persistence of herbicides and insecticides in soil is given in Table 21.2. Otganophos-phates (e.g. diazion, methyl par-athion and parathion) are perhaps the most extensively used insecticides under many agricultural systems. Biodegradation through hydrolysis of p-o-aryl bonds by Pseudomonas diminuta and Flavobacterium are considered as the most significant steps in the detoxification of organophospho-rus compounds. Organomercurials (e.g. Semesan, Panodrench, Panogen) have been practiced in agriculture since the birth of fungicides. Several species of Aspergillus, Penicillium and Trichoderma have been isolated from Semesan-treated soil.
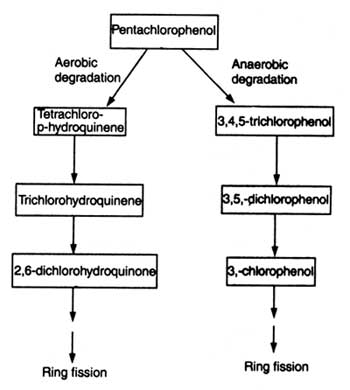
Pentachlorophenol (PCP) is a broad spectrum biocide which has been used as fungicide, insecticide, herbicide, algicide, disinfectant and antifouling agent. Bioreactors containing alginate immobilized + Polyurethane foam immobilized PCP degrading Flavobacterium (ATCC39723) cells have been used to remove PCP from contaminated water. Absorption of PCP by Polyurethane immobilized matrix plays a role in reducing the toxicity of PCP. Flavobacterium removed and detoxified PCP (Zhong -Cheng, 1994). In other experiment P. chrysosporium enzyme (ligninase) has been found to dehalogenate PCP. Steps of PCP degradation has been shown in Fig. 21.7.
| Biocides | Time taken for 75-100% disappearance |
| A. Chlorinated insecticides | |
| DDT (l,l,l-trichloro-2,2-bis-(p-chlorophenyl) ethane) | 4 years |
| Aldrin | 3 years |
| Chlordane | 5 years |
| Heptachlor | 2 years |
| Lindane (hexachloro-cyclohexane) | 3 years |
| B. Organophosphate insecticides | |
| Diazinon | 12 years |
| Malathion | 1 week |
| Parathion | 1 week |
| C. Herbicides | |
| 2,4-D (2,4-dichlorophenoxyacetic acid) | 4 weeks |
| 2,4,5-T | 30 weeks |
| Atrazine | 40 weeks |
| Simazine | 48 weeks |
| Propazine | 1.5 years |
Gene Manipulation of Pesticide-degrading Microorganisms
Day-by-day the number of xenobiotic-degrading microorganisms is increasing. However, pesticide-degrading genes of only a few microorganisms have been characterized. Most of genes responsible for catabolic degradation are located on the chromosomes, but in a few cases these genes are found on plasmids or transposons. Chakrabarty and Gunsalus (1971) found that camphor degrading genes of Pseudomonas putida are located on plasmid.
For the first time pesticide degradation through plasmid mediated genetically engineered microorganism was reported by Chakrabarty et al (1981). Nagata et al (1993) have also cloned and sequenced two genes involved in early steps of Y-HCH degradation in UT26. The linAgene encodes Y-HCH dehydrochlorinase which converts Y-HCH to 1,2,4TCB via Y-PCCH and 1,4-TCDN. The linBgene encodes 1,4-TCDN chlorohydrolase which converts 1,4-TCDN to 2,4-DDOL via 2,4,5-DNOL. This gene is a member of the haloalkanedehalogenase family with a broad range specificity for substrate. The genetically engineered P. putida comprises of both linA and linB genes (Nagata et al, 1993).