Microbial Leaching (Bioleaching, Biomining)
Thus, biomining is, economically sound hydrometallurgical process with lesser environmental problem than conventional commercial application. However, it is an inter-disciplinary field involving metallurgy, chemical engineering, microbiology and molecular biology. It has tremendous practical application. In a country like India biomining has great national significance where there is vast unexploited mineral potential (Mogal and Desai, 1992).
The most commonly used microorganisms for bioleaching are Thiobacillus thiooxidans and T.ferrooxidans. The other microorganisms may also be used in bioleaching viz., Bacillus licheniformis, B. luteus, B. megaterium, B. polymyxa, Leptospirillum ferrooxidans, Pseudomonas fluorescens, Sulfolobus acidocaldarius, Thermothrix thioparus, Thiobacillus thermophilica, etc.
Chemistry of Microbial Leaching
T. thiooxidans and T. ferrooxidans have always been found to be present in mixture on leaching dumps. Thiobacillus is the most extensively studied Gram-negative bacillus bacterium which derives energy from oxidation of Fe2+ or insoluble sulphur. In bioleaching there are two following reaction mechanisms:
Direct Bacterial Leaching
In direct bacterial leaching a physical contact exists between bacteria and ores and oxidation of minerals takes place through several enzymatically catalyzed steps. For example, pyrite is oxidized to ferric sulphate as below:
T. ferrooxidans |
||
| 2FeS2 + 7O2 + 2H2O | 2FeSO4 + 2H2SO4 |
Indirect Bacterial Leaching
In indirect bacterial leaching microbes are not in direct contact with minerals but leaching agents are produced by microorganisms which oxidize them.
FeS2 + Fe2(SO4)
2S° + 3O2 + 2H2O
Oxidation of ferrus (Fe2+) to ferric (Fe3+) by T. ferrooxidans at low pH is given below:
T. ferrooxidans |
||
| 4FeSO4 + 2H2SO4 + O2 | 2Fe2(SO4)3 + 2H2O |
There are three commercial methods used in leaching:
(i) Slope Leaching. About 10,000 tonnes of ores are ground first to get fine pieces. It is dumped in large piles down a mountain side leaching dump. Water containing inoculum of Thiobacillus is continuously sprinkled over the pile. Water is collected at bottom. It is used to extract metals and generate bacteria in an oxidation pond.
(ii) Heap Leaching. The ore is dumped in large heaps called leach dump. Further steps of treatment are as described for slope leaching.
(iii) In situ Leaching. In this process ores remain in its original position in earth. Surface blasting of rock is done just to increase permeability of water. Thereafter, water containing Thiobacillus is pumped through drilled passage to the ores. Acidic water seeps through the rock and collects at bottom. Again from bottom water is pumped, mineral is extracted and water is reused after generation of bacteria.
Examples of Bioleaching
Bioleaching has been discussed with copper, uranium, gold, silver and silica.
Copper Leaching
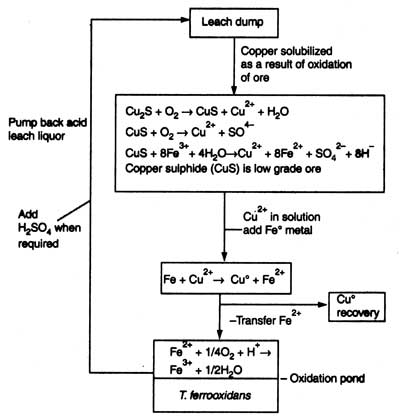
Throughout the world copper leaching plants have been widely used for many years. It is operated as simple heap leaching process or combination of both heap leaching and in situ leaching process. Dilute sulphuric acid (pH 2) is percolated down through the pile. The liquid coming out of the bottom of pile reach in mineral. It is collected and transported to precipitation plant, metal is reprecipitated and purified. Liquid is pumped back to top of pile and cycle is repeated. For removal of copper the ores commonly used are chalcocite (Cu2S), chalcopyrite (CuFeS2) or covellite (CuS). Several other metals are also associated with these ores. Chalcocite is oxidized to soluble form of copper (Cu2+) and covellite by T. ferrooxidans.
Cu2S + O2
Covellite is oxidized to copper sulphate chemically or by bacteria.
2CuFeS2 + 8½ O2 + H2SO4
Thereafter, strictly chemical reaction occurs which is the most important reaction in copper leaching.
CuS + 8Fe3+ + 4H2O
Copper is removed as below:
Fe2+ + Cu2+
Fe2+ is transferred in oxidation pond
T. ferrooxidans |
||
| Fe2+ + ¼O2 + H+ | Fe3+ + ½O2 |
The Fe3+ ions produced is an oxidation of ores; therefore, it is pumped back to pile. Sulphuric acid is added to maintain pH. An outline of microbial leaching of copper is shown in Fig. 21.9. Microbial leaching of copper has been widely used in the USA. Australia, Canada, Mexico, South Africa and Japan. In the USA 200 tonnes of copper is recovered per day.
Uranium leaching is more important than copper, although less amount of uranium is obtained than copper. For getting one tonne of uranium, a thousand tonne of uranium ore must be handled. In situ uranium leaching is gaining vast acceptance. However, uranium leaching from ore on a large scale is widely practiced in the USA, South Africa, Canada and India.
Insoluble tetravalent uranium is oxidized with a hot H2SO4/Fe3+ solution to make soluble hexavalent uranium sulfate at pH 1.5-3.5 and temperature 35°C (Crueger and Crueger, 1984).
UO2 + Fe2(SO4)3
Uranium leaching is indirect process. T. ferrooxidans does not directly attack on uranium ore, but on the iron oxidant. The pyrite reaction is used for the initial production of Fe3+ leach solution.
| T. ferrooxidans | ||
| 2FeS + H2O +7½ O2 | Fe2(SO4)3 + H2SO4 |
Gold and Silver Leaching
Today's microbial leaching of refractory precious metal ores to enhancegold and silver recovery is one of the most promising applications. Gold is obtained through bioleaching of arsenopyrite/pyrite ore and its cyanidation process. Silver is more readily solubilized than gold during microbial leaching of iron sulfide.
Magnesite, bauxite, dolomite and basalt are the ores of silica. Mohanty et al (1990) isolated Bacillus Ucheniformis from magnesite ore deposits. Later it was shown to be associated with bioleaching, concomitant mineralysis and silican uptake by the bacterium. It was concluded that silican uptake was restricted adsorption of bacterial cell surface rather than internal uptake through the membrane. The bioleaching technology of silica magnesite by using B. licheniformis developed at Bose Institute, Calcutta is being used for the first time for commissioning a 5 billion tonnes capacity of pilot plant at Salem Works of Burn, Standard Co. Ltd, Tamil Nadu, in collaboration with the Department of Biotechnology, Govt of India (Haider et al., 1994).