In vitro Androgenesis
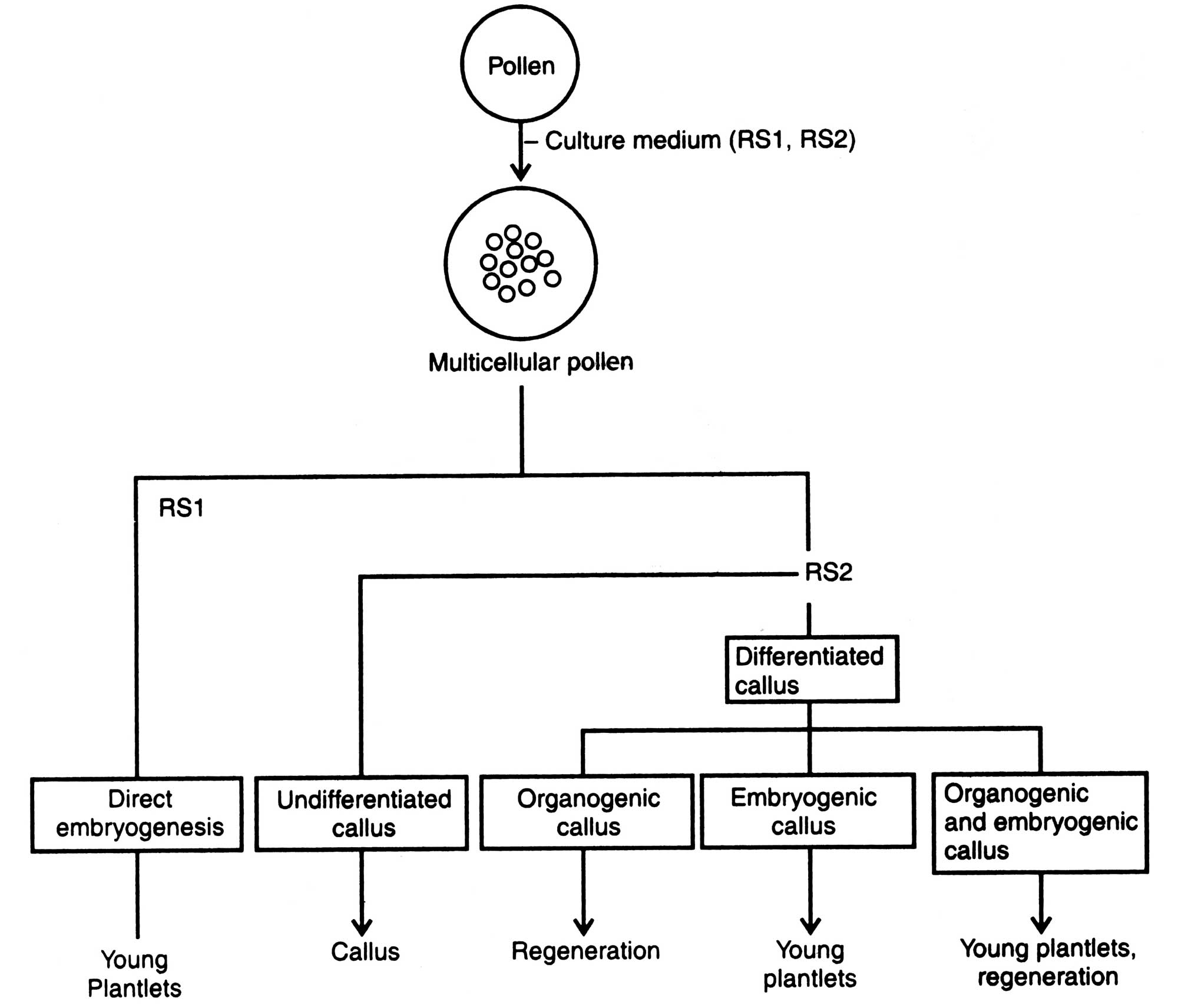
Methods of in vitro androgenesis is given in Fig. 8.10. Pollen grains are isolated from excised anther and extracted in a beaker. Well collected pollens are washed properly and centrifuged and decanted. They are inoculated in liquid medium, then subcultured in solid MS medium. In culture pollen grains can be induced to produce callus or embryo from which whole plants are regenerated in one month. This technique is most successful in Brassica, Datura, Petunia, etc. Mainly there are two ways of in vitro morphogenesis of pollen grains, direct and indirect (Fig. 8.10).
- Direct androgenesis.
Direct androgenesis is also called pollen derived embryogenesis. Here pollen directly acts as a zygote and, therefore, passes through various embryogenic stages similar to zygotic embryogenesis. When pollen grains has reached globular stage of embryo, wall of pollen is broken and embryo is released. The released embryo develops cotyledons, then the plants. Direct androgenesis is very common in many plants of the family Solanaceae and Brassicaceae (Fig. 8.10) (Prakash and Giles, 1987). - Indirect androgenesis.
In indirect androgenesis the pollen grains, instead of normal embryogenesis, divide erratically to develop callus (Fig. 8.10). Indirect androgenesis has been found in barley, wheat, Vitis, coffea, etc. Possibility of pollen morphology from the dividing pollen varies. The haploid callus, embryo or plantlets may originate from (i) the continued division of vegetative cells of the pollen (the generative cell soon degenerate), (ii) multiple division of generative cells (non-vegetative cell), and (Hi) division products of both generative and vegetative cells (Sangwan, 1981).