Assay of Tumorigenicity in Nude Mice
Tumorigenicity is defined as the ability of viable cultured cells to give rise to progressively growing tumor nodules, showing viable and mitotically active cells, in immunologically nonresponsive animals over a limited observation period (WHO, 1987). It is, however, no absolute property in a cell line, but will always be defined by the assay in which it is tested. In order to ensure reproducibility, it is therefore very important that the cells tested are from the same batch and that the animals used are syngenic. The only way of testing whether a cell line has achieved all the characteristics needed for solid tumor growth is by testing its ability to form such tumors in vivo. In order to rule out the interference of the immune defense of the recipient, testing must take place in a host organism that will not reject the transplant. If the cells being tested are originally from mice or rats, syngenic animals can be used as recipients of cell inocula. Otherwise, immune-deficient animals that are incapable of recognizing and rejecting nonself tissue are used. The nude mouse is ideal in this respect in that its immune defect affects thymic development, resulting in a lack of functional T lymphocytes in the animals (Rygaard, 1973). Therefore, a large proportion of mammalian tumors can be transplanted to nude mice without being rejected. If a cell line will not produce tumors in nude mice, it will sometimes help to mix irradiated mouse or human fibroblasts 1:1 with the tumor cell inoculum (Wilson et al., 1984). The severe combined immune deficiency (SCID) mouse, in which both T and B lymphocyte maturation are impaired due to a deficiency in a gene coding for recombinase, which participates in the recombination of genes coding for T and B lymphocyte antigen receptors, can also be used in this kind of assay (Bosma et al., 1983). Some reports say that SCID mice will let some cell lines form tumors even though they are not tumorigenic in nude mice (Xie et al., 1992).
Angiogenesis, the ability to induce blood vessel formation, is characteristic of cancer cells. The angiogenic potential of a cell line can be assayed by implanting tumor cells at the edge of rabbit cornea or by incubating it with tumor cell extracts and observing the formation of blood vessels. Implantation into the chorioallantroic membrane of chicken embryos followed by observation of the formation of blood vessels is also used for assaying angiogenesis (Folkman, 1985). Invasiveness is another characteristic of tumor cells, which can be assayed in in vitro setups.
II. MATERIALS AND INSTRUMENTATION
Mice used are 6- to 8-week-old Balb/cA nu/nu females. They are from Taconic M&B, Ry, Denmark, and are kept at our institute for 1 week before experiments are initiated. They are fed autoclaved water and sterilized food pellets and kept in Macrolon II cages under SPF conditions.
All plasticware used for tissue culture and harvest of cells is from Greiner GmbH. Tissue culture medium is RPMI 1640 with UltraGlutamin (BioWhittaker, Cat. No. 12-702F/U1). The trypsin-EDTA solution (Cat. No. 17-161E) and phosphate-buffered saline (PBS) without Ca2+ and Mg2+ (Cat. No. 17-516F) are also from BioWhittaker. Syringes for injection of tumor cells are 1-ml Luer tuberculin from Once (Cat. No. 1202) fitted with a 0.5 × 16 Terumo Neolus needle (Cat. No. NN-2516R).
III. PROCEDURES
A positive and a negative control cell line should always be included in an assay of tumorigenicity in order to ensure that the procedure has been carried out correctly. HeLa cells (American Type Culture Collection CLL-2) can be used as positive controls, and MRC- 5 cells (American Type Culture Collection CLL-171) can be used as negative controls.
A. Preparation of Cells
Solutions
- Tissue culture medium: Prepare the same cell culture medium for each of the cell lines tested that you would normally use for propagation in vitro. For this experiment, we use RPMI 1640 with 50ml/500ml of heat-inactivated calf serum (heat inactivate in a water bath at 56°C for 30min) and penicillin/streptomycin to a final concentration of 100 IU/ml penicillin and 10mg/ml streptomycin.
- PBS without Ca2+ and Mg 2+
- Trypsin-EDTA solution
Steps
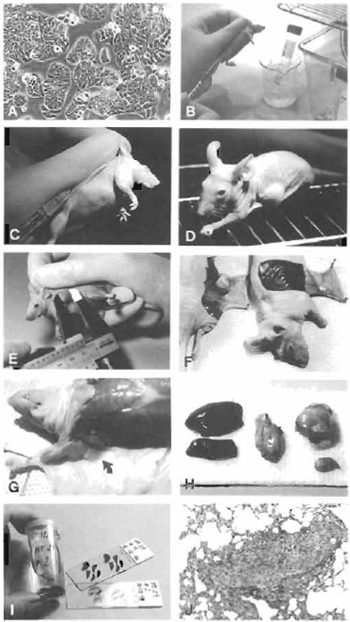 |
| FIGURE 1 (A) Subconfluent cells (colon adenocarcinoma HT29) ready to be harvested. (B) Cells kept on ice, ready to be injected. (C) Subcutaneous injection of 0.2 ml cell suspension. (D) Mouse with tumor growth. (E) Measurement of tumor. (F) Autopsy after 21 days. Mouse with and without tumor growth. (G) Axillary lymph node (arrow). (H) Organs removed for fixation in formalin. (I) Fixed tissue and histological preparations. (J) Lung metastasis (objective ×). |
- Expand the cell line(s) to be tested for tumorigenicity and the two control cell lines in culture until an appropriate number of cells has been obtained. At least 10 mice should be used for each cell line. Each mouse must receive 107 cells. Harvest the cells when they are subconfluent and thus still in the logarithmic growth phase (Fig. 1A).
- The harvest of cells from culture flasks depends on whether they grow in suspension or adhere to the flask. If cells grow adherently, they can be removed either by scraping with a cell scraper or by trypsination for 5-10min at 37°C (in an incubator). Prior to adding trypsin, remove culture medium and wash cells with 10ml PBS without calcium and magnesium in order to remove any traces of serum that will otherwise inhibit the action of trypsin.
- After removal from the flask, centrifuge the cells for 5 min at 200g, discard the supernatant, and resuspend the cells in 10 ml PBS or culture medium without antibiotics and serum. Centrifuge again at 200g for 5min. Discard supernatant and resuspend cells in 10ml PBS or medium as described earlier.
- Dilute a small sample (e.g., 0.5 ml) of the cell suspension 1:10 in PBS or medium. Dilute this solution 1:1 with 0.4% trypan blue. Let the suspension stand for 2-5 min and then count cells in a hemocytometer. Keep the rest of the cells on ice while preparing and counting the sample. Calculate the number of viable cells per milliliter of the original suspension as follows: viable cells in larges squares counted/number of large squares counted × dilution of cells × 104.
- Adjust the concentration of the cell suspension to be inoculated to 5 × 107/ml viable cells in serum-free culture medium or PBS. This may include one more step of centrifugation and resuspension in a suitable volume. Always make sure to have an excess of cell suspension as some of it will be trapped in the syringe. An extra 0.5 ml for 10 mice to be injected is sufficient.
- Keep cells on ice until use and be sure to inoculate as soon as possible after the adjustment of concentration (Fig. 1B).
Steps
- Inject 0.2 ml of the cell suspension subcutaneously in the right lateral aspect of the thoracic wall (Fig. 1C).
- Mice should be observed daily and inspected for tumor growth at least three times a week (Fig. 1D).
C. Examination of Recipients and Tumors
Solution
Prepare Lillies fixative (see Section II) and store at room temperature.
- Tumors are measured in two perpendicular dimensions (Fig. 1E). After 21 days of observation, sacrifice mice and autopsy immediately. Inspect the inoculation site from the deep aspect and excise (Fig. 1F). In order to describe the metastatic potential of the cell line tested, excise the ipsilateral axillary lymph node (Fig. 1G), part of the spleen, one-third of the liver, and the inferior lobe of the left lung for histological examination (Fig. 1H).
- Fix tissues in buffered formalin (Lillies fixative), embed in paraffin, and section in 5-µm sections. Stain with hematoxylin and eosin and study under a light microscope (Fig. 1I). Histological examination enables the investigator to see if the tumor has retained the morphological characteristics of the primary tumor from which the cell line was established and whether it gives rise to metastases (Fig. 1J).
Reports of macroscopic and microscopic findings should be given for each cell line. Macroscopic findings include the conditions of mice during the test period and macroscopic tumor growth, if any. The macroscopic appearance of the tumor-inoculation site at autopsy should also be reported. Microscopic findings should include a description of the tumor tissue or the inoculation site if no tumor appears. Metastases, location, and frequency should be reported.
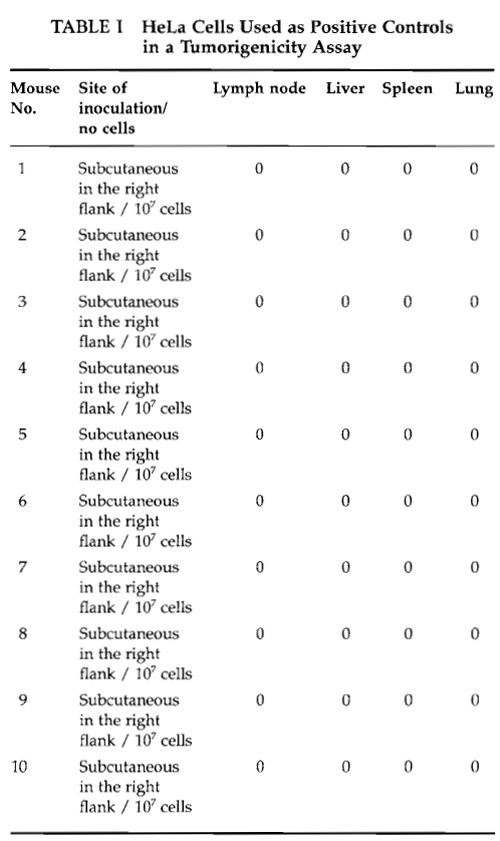
An example of an observation table is given in Table I for HeLa cells used as a positive control in a tumorigenicity assay carried out in nude Balb/cA mice.
 |
E. Conclusions
According to the World Health Organization, the positive control cell (HeLa) should give rise to progressively growing tumors in 9 out of 10 mice if the assay is carried out correctly. Furthermore, when a histopathological examination of tumor nodules is performed, mitotic activity must be detected in the tumor tissue.
IV. PITFALLS
It is of the utmost importance for the interpretation of results that the cell lines tested be free of mycoplasma infection, as mycoplasma can alter their ability to form tumors (Fogh, 1973). If there is not a routine of regular mycoplasma testing in the cell culture laboratory, a mycoplasma test should be performed and a negative outcome ensured before cell lines are used in a tumorigenicity assay.
References
Bosma, G. C., Custer, R. P., and Bosma, M. J. (1983). A severe combined immunodeficiency in the mouse. Nature 301, 527-530.
Cheng, J. Q., Ruggeri, B., Klein, W. M., Sonoda, G., Altomare, D. A., Watson, D. K., and Testa, J. R. (1996). Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. USA S 93, 3636-3641.
Fogh, J. (ed.) (1973). "Contamination in Tissue Culture." Academic Press, New York.
Folkman, J. T. (1985). Tumor angiogenesis. Adv. Cancer Res. 43, 175-203.
Gurtsevitch, V. E, and Lenoir, G. M. (1985). Tumorigenic potential of various Burkitt's lymphoma cell lines in nude mice. In "Immune- Deficient Animals in Biomedical Research" (J. Rygaard et al., eds.), pp. 199-203. Karger, Basel.
Hamburger, A. W., and Salmon, S. E. (1977). Primary bioassay of human tumor stem cells. Science 197, 461-463.
Iiaza, T., Momiki, S., Bauer, B., Caamano, J., Metcalf, R., Lechner, J., Harris, C. C., and Klein-Szanto, A. J. (1993). Invasive tumors derived from xenotransplanted, immortalized human cells after in vivo exposure to chemical carcinogens. Carcinogenesis 14, 1789-1794.
Nicolson, G. L. (1976). Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim. Biophys. Acta 458, 1-72.
Shimizu, T., Kato, M. V., Nikaido, O., and Suzuki, E (1995). A specific chromosome change and distinctive transforming genes are necessary for malignant progression of spontantous transformation in cultured Chinese hamster embryo cells. Jpn. J. Cancer Res. 86, 546-554.
Sun, Y., Kim, H., Parker, M., Stetler-Stevenson, W. G., and Colburn, N. H. (1996). Lack of suppression of tumor cell phenotype by overexpression of TIMP-3 in mouse JB6 tumor cells: Identification of a transfectant with increased tumorigenicity and invasiveness. Anticancer Res. 16, 1-17.
Wilson, E. L., Gärtner, M., Campbell, J. A. H., and Dowdle, E. B. (1984). Growth and behaviour of human melanomas in nude mice: Effect of fibroblasts. In "Immune-Deficient Animals" (B. Sordat, ed.), pp. 357-361. Karger, Basel.
World Health Organization (WHO) (1987). "Requirements for Continuous Cell Lines Used for Biologicals Production." WHO Technical Report Series, No. 745, Annex 3.
Xie, X., Br6nner, N., Jensen, G., Albrectsen, J., Gotthardsen, B., and Rygaard, J. (1992). Comparative studies between nude and scid mice on the growth and metastatic behavior of xenografted human tumors. Clin. Exp. Metast. 10, 201-210.




