Endothelial Cell Invasion Assay
Angiogenesis is defined as the sprouting of new capillaries from preexisting blood vessels (Folkman and Shing, 1992). The formation of new blood vessels occurs in a variety of normal and pathologic conditions. During embryogenesis or wound healing, neovascularization is the result of a balance of stimulatory and inhibitory angiogenic factors. Normally this balance is strictly controlled. However, during the development of many diseases, including inflammation, retinopathies, and cancer metastasis, the angiogenesis controlling mechanisms may fail and result in formation of a pathologic capillary network (Folkman, 1992).
During vascular assembly, endothelial cells respond to a variety of extracellular growth factors and different molecules involved in cell-cell and cell-matrix interactions (Gale and Yancopoulos, 1999). Signaling molecules, more commonly associated with neuronal development, also play an important role in capillary formation during angiogenesis (Wang et al., 1998; Soker et al., 1998).
Another advantage is that in this assay, cultivated endothelial cell lines can be used, which can give more standard results than the use of primary cultures, or organ cultures.
Growth medium: Dulbecco's modified Eagle's medium (DMEM) with a high concentration of glucose (Cat. No. 31966-021,GIBCO) supplemented with fetal bovine serum (FBS, Cat. No. F 7524, Sigma) at 10%, 100× penicillin/streptomycinx (Cat. No. 15140-148, GIBCO).
PBS(-) Ca2+ and Mg2+-free Dulbecco's phosphatebuffered saline (PBS, 2.7mM KH2PO4, 8.1mM Na2HPO4, 137 mM NaCl), sterile.
Trypsin-EDTA solution (Cat. No. 25200-056, GIBCO)
HEPES buffer solution 1M (Cat. No. 15630-049, GIBCO)
Stock of Matrigel - basement membrane matrix (BD Biosciences, Cat. No. 354234). Matrigel is supplied frozen and is stored at -70°C.
25-cm2 tissue culture flasks, 48-well tissue culture plates, Pasteur pipettes, 10-ml pipettes (sterile), and syringe needles (23 gauge).
III. PROCEDURES
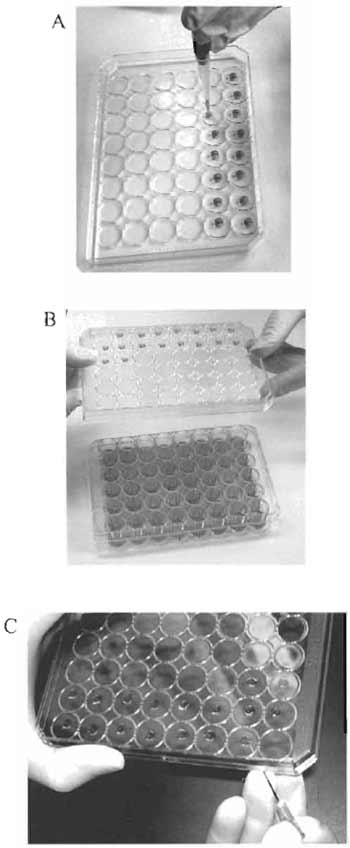 |
| FIGURE 1 Culturing cells in the hanging drops. (A) Plating of the cell suspension in the middle of the inner side of the lid. (B) Turning over the lid with the hanging drops. (C) Transferring the cell "clump" with a syringe needle. |
- For culture in 75-cm2 flasks, suspend murine microvascular endothelial SVEC4-10 cells in culture medium at a concentration of 2 × 105 cells/ml and plate out 2-4ml of cell suspension/flask. Place the flasks in a 37°C tissue culture incubator and incubate in an atmosphere of 5% CO2 overnight.
- The next day, examine cells under an inverted microscope fitted with phase-contrast objectives. Choose the flasks with the cell density >80% of confluence.
- Remove media and wash the cells with 10ml PBS(-).
- Replace the PBS with 1 ml of trypsin-EDTA and incubate at 37°C for approximately 1 min. Cell rounding should be observed in the inverted microscope. When the cells are rounded, detach them by strong agitation. Add 10 ml of culture medium supplemented with 10% FBS to the flask, pipette the cells up and down five times, and transfer contents to a 15-ml centrifuge tube.
- Centrifuge the cells at 800g for 5min at room temperature. Resuspend the cells in 10ml DMEM/ FBS. Count an aliquot of the cell suspension with the Coulter counter or in a hemocytometer.
- Centrifuge the cells at 800g for 5min at room temperature.
- Resuspend the cells in DMEM supplemented with 20% FBS, 1M HEPES, pH 7.5, at a concentration of 3 × 106 cells/ml.
- Dispense 1.0ml of DMEM/FBS into each well of a 48-well tissue culture plate.
- Turn the lid of the plate upside down. Plate out 0.02 ml of the cell suspension in the middle of the inner side of the lid. The cell suspension should form a drop (Fig. 1A).
- Carefully turn over the lid and place it on the plate. The drops of the cell suspension will hang over the media in the wells (Fig. 1B).
- Incubate plates at 37°C in a tissue culture incubator. During overnight incubation, cells form a dense "clump" at the bottom of the drop.
- Defreeze the necessary amount of Matrigel overnight at 4°C with rotation.
- The next day, examine all the wells under a stereomicroscope and select those that have the most compact, well-formed "clump" of cells.
- Place Matrigel on ice. Add 5 × DMEM and FBS to obtain a final concentration of 1× DMEM and 10% FBS. Keep the mixture on ice.
- Place a fresh 48-well plate on ice. Dispense 0.15ml/well of Matrigel/mix into the plate. Incubate the plate at 37°C in an incubator for 3-4 hours. The Matrigel should solidify.
- Carefully lift the lid of the plate with hanging drops, stick the cell "clump" to the tip of a syringe needle, and transfer the "clump" on the surface of the solidified Matrigel in the 48-well tissue culture plate (Fig. 1C).
- Cover the cell "clump" with 0.01 ml of Matrigel/ mix and incubate the plate for 15min at 37°C in an incubator.
- Add 0.3ml of DMEM/FBS in each well and return the plate to the incubator. The cells will remain viable for several days.
IV. COMMENTS
Using the protocol described in this assay, it is possible to demonstrate the effects of compounds on the ability of endothelial cell to form capillary-like tubes in vitro. We have also used this protocol to cocultivate endothelial cells with other cell types and to study the influence of the molecules produced by these cells on the ability of endothelial cells to form capillaries (Fig. 2D).
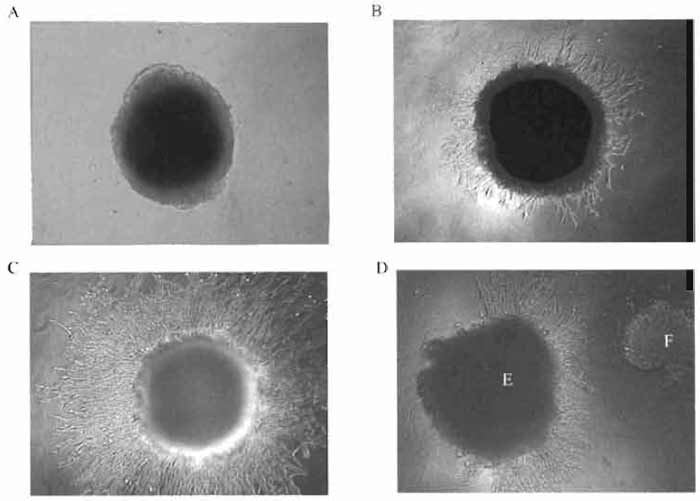 |
| FIGURE 2 Mouse endothelial cells SVEC4-10 migrating from a dense "clump" of the cells into Matrigel containing an angiogenic compound: (A) 0 h of cultivation, (B) 24h, (C) 48 h, and (D) chemotactic activity of an angiogenic compound. E, endothelial cells; F, tumor cells. |
- To obtain maximum viability of cells growing in the hanging drops, avoid long exposure of the cells to trypsin during harvesting cells from the 75-cm2 flasks.
- Choose only well-formed, round-shaped, dense cellular "clumps."
- When lifting the lid with the hanging drops, caution must be exercised to avoid disrupting the cell "clumps."
References
Folkman, J. (1986). How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial award lecture. Cancer Res. 46, 467-473.
Folkman, J. (1992). The role of angiogenesis in tumor growth. Semin. Cancer Biol. 3, 65-71.
Folkman, J., and Shing (1992). Angiogenesis. J.Biol.Chem. 267, 10931-1093.
Gale, N., and Yancopoulos, G. D. (1999). Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins and ephrins in vascular development. Genes Dev. 13, 1055-1066.
Gumkowski, E, Kaminska, G., Kaminski, M., Morrissey, L. M., and Auerbach R. (1991). Heterogeneity of mouse vascular endothelium: in vitro studies of lymphatic, large blood vessels and microvascular endothelial cells. Blood Vessels 24, 11-23.
Kråling, B. M., Jimenez, S. A., Sorger, T., and Maul, G. G. (1994). Isolation and characterization of microvascular endothelial cells from the adult human dermis and from skin biopsies of patients with systemic sclerosis. Lab. Invest. 71, 745-754.
Lamszus, K., Schmidt, N. O., Ergun, S., and Westphal, M. (1999). Isolation and culture of human neuromicrovascular endothelial cells for the study of angiogenesis in vitro. J. Nerosci. Res. 55, 370-381.
Madri, J. A., Pratt, B. M., and Tucker, A. M. (1988). Phenotipic modulation of endothelial cells by transforming growth factorq3 depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 106, 1375-1384.
Muthukkaruppan, V. R., Shinners, B. L., Lewis, R., Park, S-J., Baechler, B. J., and Auerbach, R. (2000). The chick embryo aortic arch assay: A new, rapid, quantifiable in vitro method for testing the efficacy of angiogenic and anti-angiogenic factors in a threedimensional, serum-free organ culture system. Proc. Am. Assoc. Cancer Res. 41, 65.
Nicosia, R. E, and Ottinett, A. (1990). Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 63, 115-122.
Springhorn, J. P., Madri, J. A., and Squinto, S. P. (1995). Human capillary endothelial cells from abdominal wall adipose tissue: Isolation using an anti-PECAM antibody. in vitro Cell Dev. Biol 31, 473-481.
Wang, H. U., Chen, Z. E, and Anderson, D. J. (1998). Molecular distinction and angiogenic interaction between embryonic arteries and viens revealed by ephrin B2 and its receptor EphB4. Cell 93, 741-753.
Zetter, B. R. (1988). Endothelial heterogeneity: Influence of vessel size, organ localization, and species specificity on the properties of cultured endothelial cells. In "Endothelial Cells" (U.S. Ryan, ed.), vol. 2, pp. 63-79. V. 2. CRC, Boca Raton, FL.




