Scientific method and design of experiments
Science is a systematized body of knowledge derived from observation and experiment. Scientists carry out experiments, make observations and attempt to explain the results; these tentative explanations are called hypotheses and their validity is tested by systematically forming and rejecting alternative explanations.Some branches of chemistry are designed to provide fundamental information, rather than to test a particular hypothesis: for example, the purification and characterization of a newly discovered naturally occurring molecule. In contrast, an experiment is a contrived situation designed to test one or more hypothesis under conditions controlled by the investigator. Any hypothesis that cannot be rejected from the results of an experiment is provisionally accepted. This 'sieve' effect leaves us with a set of current explanations for our observations. These explanations are not permanent and may be rejected on the basis of a future investigation. A hypothesis that has withstood many such tests and has been shown to allow predictions to be made is known as a theory, and a theory may generate such confidence through its predictive abilities to be known as a law (Fig. 10.1).
Observations are a prelude to experimentation, but they are preconditioned by a framework of peripheral knowledge. While there is an element of luck in being at the right place and time to make important observations, as Pasteur stated, 'chance favours only the prepared mind'. A fault in scientific method is that the design of the experiment and choice of method may influence the outcome - the decisions involved may not be as objective as some scientists assume. Another flaw is that radical alternative hypotheses may be overlooked in favour of a modification to the original hypothesis, and yet just such leaps in thinking have frequently been required before great scientific advances.
No hypothesis can ever be rejected with certainty. Statistics allow us to quantify as vanishingly small the probability of an erroneous conclusion, but we are nevertheless left in the position of never being 100% certain that we have rejected all relevant alternative hypotheses, nor 100% certain that our decision to reject some alternative hypotheses was correct! However, despite these problems, experimental science has yielded and continues to yield many important findings.
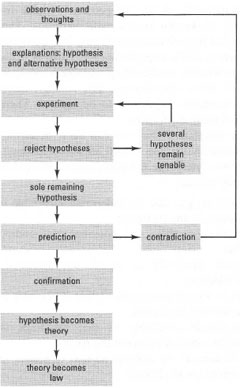 |
| Fig. 10.1 How scientific investigations proceed. |
Quantitative hypotheses involve a mathematical description of the system. They can be formulated concisely by mathematical models. Models are often useful because they force deeper thought about mechanisms and encourage simplification of the system. A mathematical model:
- is inherently testable through experiment;
- identifies areas where information is lacking or uncertain;
- encapsulates many observations;
- allows you to predict the behaviour of the system.




