Bioenergetics
An amalgamation of the term biological energetics, is the branch of biology and biochemistry that is concerned with how organisms extract energy from their environment and with how energy is used to fuel the myriad of life’s endergonic processes. Organisms may be usefully divided into two broad groups with respect to how they satisfy their need for energy. Autotrophic organisms convert energy from nonorganic sources such as light or from the oxidation of inorganic molecules to chemical energy. As heterotrophic organisms, animals must ingest and break down complex organic molecules to provide the energy for life.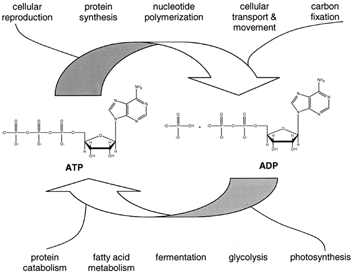
Figure 1 Central role of adenosine 5´-triphosphate (ATP) in metabolism. Catabolic (degradative) metabolism is exergonic and provides the energy needed for the synthesis of ATP from adenosine 5´-diphosphate (ADP) and inorganic phosphate (Pi). The exergonic hydrolysis of ATP in turn powers the endergonic processes of organisms.
Interconversions of forms of energy are commonplace in the biological world. In photosynthesis, the electromagnetic energy of light is converted to chemical energy, largely in the form of carbohydrates, with high overall efficiency. The energy of light is used to drive oxidation– reduction reactions that could not take place in the dark. Light energy also powers the generation of a proton electrochemical potential across the green photosynthetic membrane. Thus, electricalwork is an integral part of photosynthesis. Chemical energy is used in all organisms to drive the synthesis of large and small molecules, motility at the microscoPic and macroscoPic levels, the generation of electrochemical potentials of ions across cellular membranes, and even light emission as in fireflies.
Given the diversity in the forms of life, it might be expected that organisms have evolved many mechanisms to deal with their need for energy. To some extent this expectation is the case, especially for organisms that live in extreme environments. However, the similarities among organisms in their bioenergetic mechanisms are as, or even more, striking than the differences. For example, the sugar glucose is catabolized (broken down) by a pathway that is the same in the enteric bacterium Escherichia coli as it is in higher organisms. All organisms use adenosine 5´-triphosphate (ATP) as a central intermediate in energy metabolism. ATP acts in a way as a currency of free energy. The synthesis ofATP from adenosine 5´-diphosphate (ADP) and inorganic phosphate (Pi) is a strongly endergonic reaction that is coupled to exergonic reactions such as the breakdown of glucose. ATP hydrolysis in turn powers many of life’s processes. The central role of ATP in bioenergetics is illustrated in Fig. 1. Partial structures of several compounds that play important roles in metabolism are shown in Fig. 2. eral compounds that play important roles in Fig.2
In this article, the elements of energy metabolism will be discussed with emphasis on howorganisms satisfy their energetic requirements and on how ATP hydrolysis drives otherwise unfavorable reactions.
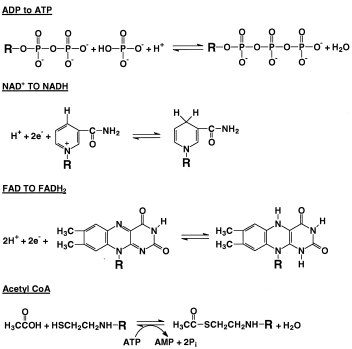 |
| Figure 2 Some important reactions in metabolism. Shown are the phosphorylation of ADP to ATP, NAD+, NADH, FAD, FADH2 acetate, CoA, and acetyl CoA. For clarity, just the parts of the larger molecules that undergo reaction are shown. NAD+, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide (reduced form); FAD, flavin adenine dinucleotide; FADH2, flavin adenine dinucleotide (reduced form); CoA, coenzyme A; AMP, adenosine monophosphate. |




