Oxidation of Fats and Oils, Major Metabolic Fuels
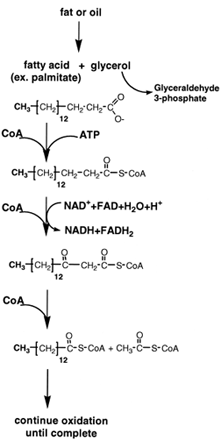 |
| Figure 9 Oxidation of fatty acids. Fats and oils are hydrolyzed to form glycerol and fatty acids. CoA derivatives of the fatty acids are oxidized in mitochondria by NAD+ and FAD to β-oxo-derivatives. CoA cleaves these derivatives to yield acetyl CoA and a fatty acid CoA molecule that is two carbons shorter. The process continues until the fatty acid has been completely converted to acetyl CoA. The acetyl moiety is oxidized in the citric acid cycle to CO2 and water. The complete oxidation of a fatty acid of about the same molecular weight of glucose yields four times more ATP than that of glucose. |
Fats and oils are ubiquitous biological molecules that are major energy reserves in animals and developing plants. Fats and oils are esters of glycerol, a three-carbon compound with hydroxyl groups on all three carbons, and carboxylic acids with long hydrocarbon chains. The most common fats and oils contain fatty acids with straight chains with an even number of carbon atoms. Most often, the total number of carbons in a fatty acid in a triglyceride ranges from 14 to 18. The difference between a fat and an oil is simply melting temperature. Oils are liquid at room temperature, whereas fats are solid. Familiar examples are olive oil and butter.
The most significant reason for this difference in melting temperatures between fats and oils is the degree of unsaturation (double bonds) of the fatty acids they contain. The introduction of double bonds into a hydrocarbon chain causes perturbations in the structure of the chain that decrease its ability to pack the chains closely into a solid structure. Olive oil contains far more unsaturated fatty acids than butter does and is thus a liquid at room temperature and even in the cold.
Regardless of the physical properties of triglycerides, they are the long-term energy reserves of higher organisms. Consider the fact that the complete oxidation of triglycerides to CO2 and water yields 9 kcal/g, whereas that of the carbohydrate storage polymers, starch and glycogen, yields just 4 kcal/g. When it is also remembered that fats and oils shunwater, but glycogen and starch are more hydrophilic, triglycerides have an additional advantage over the glucose polymers as deposits of potential free energy. As hydrophobic moieties, fats and oils require less intracellular space than that required by the glucose polymers.
Thefirst stepinthebreakdownof triglycerides (Fig. 9) is their conversion by hydrolysis to their components, glycerol and fatty acids. Glycerol is a close relative of the threecarbon compounds involved in the catabolism of glucose and may be completely oxidized to CO2 and water by glycolysis and the tricarboxylic acid cycle.
The fatty acids are first converted to CoA derivatives at the expense of the hydrolysis of ATP and then transported into mitochondria where they are broken down sequentially, two carbon units at a time, by a pathway known as β-oxidation (see Fig. 9). The fatty acyl CoA derivatives undergo oxidation at the carbon that is β to the carboxyl carbon from that of a saturated carbon–carbon bond to that of an oxo-saturated carbon bond. Enzymes that contain FADor use NAD+ as the electron acceptors catalyze these reactions. As is the case in the oxidation of carbohydrates, the NADH and FADH2 generated by the β-oxidation of fatty acids are converted to their oxidized forms by the mitochondrial electron transport chain, which results in the formation of ATP by oxidative phosphorylation.
Once β-oxidation is complete, the terminal two carbons of the fatty acid chain are then released as acetyl CoA. Oxidation and cleavage of the fatty acid continue until it is entirely converted to acetyl CoA. The conversion of a saturated fatty acid with 18 carbon atoms to 9 acetyl CoA produces 8 NADH and 8 FADH2. The acetyl CoA is burned by the citric acid cycle to generate more ATP. The high caloric content of fats pays off to cells in the yield of ATP.




