Oxidation of Pyruvate: the Citric Acid Cycle
In both cases two electrons are transferred to oxygen, so that the n in Eq. (1) is equal to 2. Under standard conditions, the oxidation of 1 mol of NADH by oxygen liberates close to 53 kcal, whereas the ΔG0' for that of FADH2 is −38 kcal/mol. These two strongly exergonic reactions provide the energy for the endergonic synthesis of ATP.The details of carbon metabolism in the citric acid cycle are beyond the scope of this article. In brief, pyruvate is first oxidatively decarboxylated to yield CO2, NADH, and an acetyl group attached in an ester linkage to a thiol on a large molecule, known as coenzyme A, or CoA. (See Fig. 2.) Acetyl CoA condenses with a four-carbon dicarboxylic acid to form the tricarboxylic acid citrate. Free CoA is also a product (Fig. 6). A total of four oxidation– reduction reactions, two of which are oxidative decarboxylations, take place, which results in the generation of the three remaining NADH molecules and one molecule of FADH2. The citric acid cycle is a true cycle. For each two-carbon acetyl moiety oxidized in the cycle, two CO2 molecules are produced and the four-carbon dicarboxylic acid with which acetyl CoA condenses is regenerated.
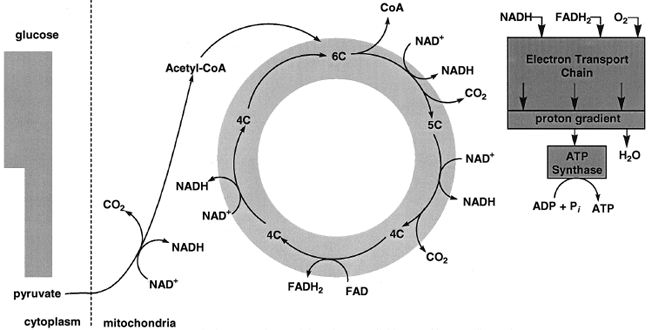 |
| Figure 6 A view of the oxidation of pyruvate. The oxidation of pyruvate generates three CO2, four NADH, and one FADH2. The oxidation of NADH and FADH2 by the mitochondrial electron transport chain is exergonic and provides most of the energy for ATP synthesis. |
The mitochondrial inner membrane (Fig. 7) contains proteins that act in concert to catalyze NADH and FADH2 oxidation by molecular oxygen. [See reactions (2) and (3) above.] These reactions are carried out in many small steps by proteins that are integral to the membrane and that undergo oxidation–reduction. These proteins make up what is called the mitochondrial electron transport chain. Components of the chain include iron proteins (cytochromes and iron–sulfur proteins), flavoproteins (proteins that contain flavin), copper, and quinone binding proteins. 1960s that electron transport through the mitochondrial chain is obligatorily linked to the movement of protons across the inner membrane of the mitochondrion. In this way, part of the energy liberated by oxidative electron transfer is conserved in the form of the proton electrochemical potential. This potential, ΔµH+ , is the sum of contributions from the activity gradient and that of the electrical gradient:
| ⇒ Equation [4] | ΔµH+ = RT ln ([H+]a/[H+]b) + FΔφ, |
where R is the gas constant; T , the absolute temperature; a and b, the aqueous spaces bounded by the membrane; F, Faraday’s constant; and Δφ, the membrane potential. As Mitchell suggested, the mitochondrial inner membrane is poorly permeated by charged molecules, including protons. The membrane thus provides an insulating layer between the two aqueous phases it separates. Thus the transport of protons across the membrane generates an electrochemical potential. In the case of mitochondria, the membrane potential is the predominant component of the electrochemical of the proton. The total ΔµH+ in actively respiring mitochondria is on the order of −200 mV, if one uses the convention that the inside space bounded by the membrane is negative.
Electron transport from NADH and FADH2 to oxygen provides the energy for the generation of the electrochemical potential of the proton. The flow of protons down this potential is exergonic and is the immediate source of energy for ATP synthesis. The proton-linked synthesis of ATP is catalyzed by a complex enzyme called ATP synthase. Remarkably similar enzymes are located in the coupling membranes of bacteria, mitochondria, and chloroplasts, the intracellular sites of photosynthesis in higher plants. Even though the reaction that they catalyze seems relatively straightforward (see Fig. 2), the ATP synthases contain a minimum of 8 different proteins and a total of about 20 polypeptide chains.
ATP is formed in the aqueous space bounded by the mitochondrial inner membrane. This space is known as the matrix (see Fig. 7). Most of the ATP generated within mitochondria is exported to the cytoplasm where it is used to drive energy-dependent reactions. The ADP and Pi formed in the cytoplasm must then be taken up by the mitochondria. The inner membrane contains specific proteins that mediate the export of ATP and the import of ADP and Pi. One transporter catalyzes counterexchange transport of ATP out of the matrix with ADP in the cytoplasm into the matrix (Fig. 8). At physiological pH, ATP bears four negative charges, and ADP, three. Thus, the one-to-one exchange transport of ATP with ADP creates a membrane potential that is opposite in sign of that created by electrontransport- driven proton translocation. ATP/ADP transport costs energy and the direction of transport is poised by the proton membrane potential. In addition, phosphate uptake into mitochondria is coupled to the electrochemical proton potential. The phosphate translocator (see Fig. 8) catalyzes the counterexchange transport of H2PO42− and hydroxide anion (OH−). The outward movement of OH− causes acidification of the matrix, whereas the direction of proton transport driven by electron transport is out of the mitochondrial matrix and results in an increase in the pH of the matrix.
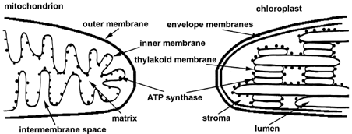 |
| Figure 7 Diagrams of the structures of mitochondria and chloroplasts. The inner membrane of mitochondria and the thylakoid membrane of chloroplasts contain the electron transport chains and ATP synthases. Note that the orientation of the inner membrane is opposite that of the thylakoid membrane. |
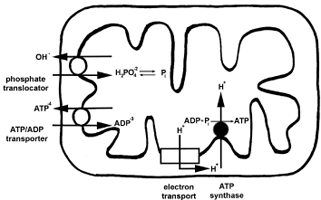 |
| Figure 8 ATP, ADP, and Pi transport in mitochondria. ATP is formed inside mitochondria. Most of the ATP is exported to the cytoplasm where it is cleaved to ADP and Pi. The mitochondrial inner membrane contains specific proteins that mediate not only ATP release coupled to ADP uptake, but also Pi uptake linked to hydroxide ion (OH−) release. |
In the total oxidation of glucose to CO2 and water, six CO2 are released and six O2 are reduced to water. For each pyruvate oxidized, four NADH and one FADH2 are generated. Since two molecules of pyruvate are derived by means of glycolysis from one molecule of glucose, a total of eight NADH and two FADH2 are formed by pyruvate oxidation. Four electrons are required for the reduction of O2 to two molecules of H2O. Thus, pyruvate oxidation accounts for the reduction of five of the six molecules of O2 in the complete oxidation of glucose. The sixth O2 is reduced to water by electrons from the NADH formed by the oxidation of triose phosphate in glycolysis.
Fermentation, or anaerobic glycolysis, yields but 2 mol of ATP per 1 mol of glucose catabolized. In contrast, complete oxidation of glucose to CO2 and water yields about 15 times more ATP. Thus, it is understandable why yeasts and some bacteria consume more glucose under anaerobic conditions than when oxygen is present.
In animals, glucose is normally completely oxidized. During strenuous exercise, however, the demand for oxygen by muscle tissues can outstrip its supply and the tissue may become anaerobic. Muscle contraction requires ATP, and rapid breakdown of glucose and its storage polymer, glycogen, takes place under anaerobiosis. Glycolysis would stop quickly if the NADH produced by the oxidation of triose phosphate were not converted back to NAD+. In muscle cells under O2-limited conditions, pyruvate is reduced by NADH to lactic acid (see Fig. 5), a source of muscle cramps during exercise. At rest, lactic acid is converted back to glucose in the liver and kidneys and returned to muscle tissues where it stored in the form of glycogen.




