Telomere Shortening: Linkage Between Telomere Length and Limited Life Span
One profound implication of the specialized telomere
structure and its synthesis is that in the absence of telomerase,
the repeat length of telomeres could not be maintained.
Telomerase is active in neonatal cells and also in
some immortal tumor cells, but is barely detectable in
diploid, terminally differentiated mammalian cells. Most
such diploid cells can multiply in
vitro in specialized culture
medium, but have a limited life span. Loss of replicative
capacity is associated with shortening of telomere repeat
lengths. Furthermore, ectopic and stable expression
of telomerase in human diploid cells by introduction of its
gene confer an indefinite reproductive life on such cells. It
is generally believed that cells will senesce if the telomere
length is reduced belowa critical level after repeated replication
of the genome.
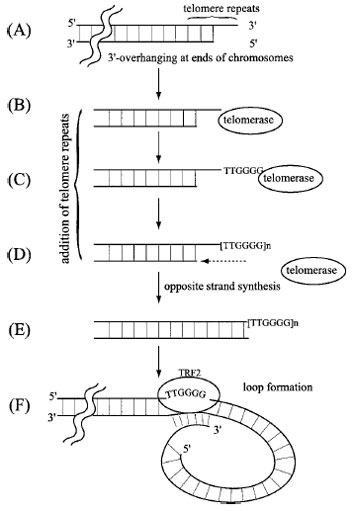 |
| FIGURE 6 A schematic description of the role of telomerase in
the maintenance of telomeres at chromosome termini. The double
lines with break represent one telomere terminus of a chromosome
in which the 5´ terminal region of the lagging strand is
unreplicated (as in Fig. 4), resulting in an overhanging 3´ terminal
region. In order to avoid shortening of this telomere sequence during
successive rounds of replication, DNA template-independent
telomerase extends the 3´ overhang by adding the telomere repeat
sequence TTGGGG as shown in (C). The template for the repeat
is an RNA present in the telomerase complex. The extended 3´
single-strand region then allows de novo initiation and filling in of
the 5´ strand (E). Finally, the 3´ overhang loops to anneal with an
internal sequence mediated by the telomere repeat factor (TRF2)
in order to protect the terminus from degradation by nonspecific
nucleases (F). |





