Phylogeny and Adaptive Radiation
Phylogeny and
Adaptive Radiation
Phylogeny
Hyman (1951) grouped Rotifera, Gastrotricha,
Kinorhyncha, Nematoda,
and Nematomorpha into a single phylum
(Aschelminthes). All of these
phyla share a certain combination of
characteristics. Hyman contended
that such evidences of relationships
were so concrete and specific that
they could not be disregarded. Nevertheless,
most authors now consider
that differences between the groups
are sufficient to merit phylum status
for each, although some accept the
concept of the Aschelminthes as a
superphylum. These phyla may well
have been derived originally from the
protostome line via an acoelomate
common ancestor (Figure 15-22).
However, sequence analysis of the
gene encoding the small subunit of
ribosomal RNA supports
a quite different phylogenetic hypothesis.* This evidence suggests that
some time after the ancestral
deuterostome diverged from the
ancestral protostome in the Precambrian,
protostomes split again into
two large groups (or superphyla):
Ecdysozoa, containing phyla that go
through a series of molts during
development, and Lophotrochozoa,
including lophophorate phyla (Lophophorate Animals ) and phyla whose larvae are
trochophore-like. Some
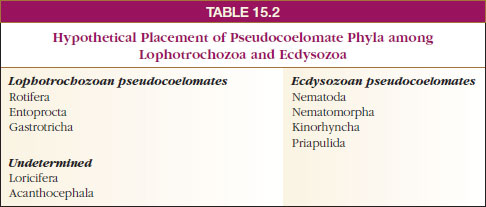
pseudocoelomates fall into Ecdysozoa;
sequences of others place them
in Lophotrochozoa, and some are yet
to be determined (Table 15-2). If this
arrangement is correct, the concept of
Aschelminthes is invalid. We will discuss
the Ecdysozoa/Lophotrochozoa
hypothesis further in Arthropods .
Loriciferans bear some similarity to
kinorhynchs, larval Priapulida, larval
nematomorphs, rotifers, and tardigrades. Although loriciferans
are poorly known, cladistic analysis
suggests that they form a sister group
to kinorhynchs and that these two
phyla together are a sister group of priapulids
(Figure 15-22). If so, we would
expect sequence analysis to place Loricifera
in Ecdysozoa.
Entoprocts were once included with phylum Ectoprocta in a phylum called Bryozoa, but ectoprocts are true coelomate animals, and many zoologists prefer to place them in a separate group. Ectoprocts are still often called bryozoans. Sequence analysis places both entoprocts and ectoprocts among lophotrochozoan phyla.
Acanthocephalans are highly specialized parasites with a unique morphology and have doubtless been so for millions of years. Any ancestral or other related group that would shed a clue to phyletic relationships of Acanthocephala is probably long since extinct. Like cestodes, acanthocephalans have no digestive tract and must absorb all nutrients across their tegument, but the tegument of the two groups is quite different in structure. Also, acanthocephalans are pseudocoelomate and show eutely, as do nematodes, although here, too, structural and developmental differences are great.
Adaptive Radiation
Certainly the most impressive adaptive radiation in this group of phyla is shown by nematodes. They are by far the most numerous in terms of both individuals and species, and they have been able to adapt to almost every habitat available to animal life. Their basic pseudocoelomate body plan, with the cuticle, hydrostatic skeleton, and longitudinal muscles, has proved generalized and plastic enough to adapt to an enormous variety of physical conditions. Free-living lines gave rise to parasitic forms on at least several occasions, and virtually all potential hosts have been exploited. All types of life cycle occur: from simple and direct to complex, with intermediate hosts; from normal dioecious reproduction to parthenogenesis, hermaphroditism, and alternation of free-living and parasitic generations. A major factor contributing to evolutionary opportunism of nematodes has been their extraordinary capacity to survive suboptimal conditions, for example, developmental arrests in many free-living and animal parasitic species and ability to undergo cryptobiosis (survival in harsh conditions by assuming a very low metabolic rate) in many freeliving and plant parasitic species.
Phylogeny
 |
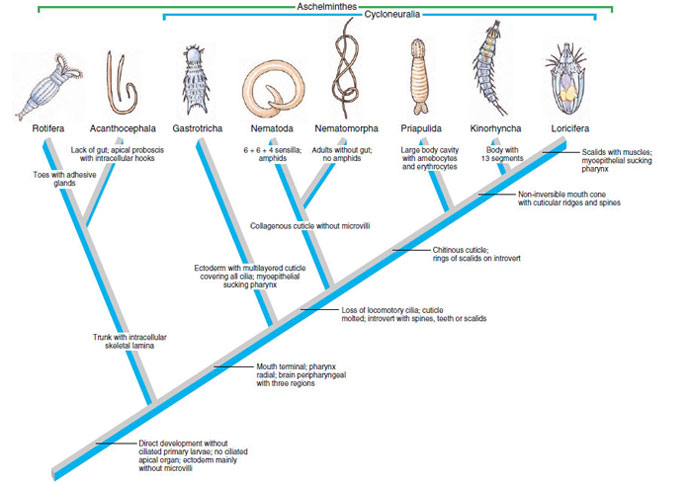 |
| Figure 15-22 Hypothetical relationships among pseudocoelomate phyla according to Nielsen (1995). Nielsen placed all phyla with a radial pharynx and nerve ring around the pharynx in a monophyletic group he called Cycloneuralia, and he moved Entoprocta, along with Ectoprocta, to form a protostome group (Bryozoa). The synapomorphy for nematodes “6 + 6 + 4 sensilla” refers to the rings of sensory papillae (sensilla) on the anterior end. The outgroup for this cladogram is Spiralia (protostomes with spiral cleavage, trophophore larva, and mesoderm from the 4d cell). If interpretation of sequence data (cited in text) is supported by further research, relationships shown here must be substantially revised because some phyla have been assigned to Lophotrochozoa (Rotifera, Gastrotricha) and others to Ecdysozoa (Nematoda, Nematomorpha, Priapulida, Kinorhyncha). |
Entoprocts were once included with phylum Ectoprocta in a phylum called Bryozoa, but ectoprocts are true coelomate animals, and many zoologists prefer to place them in a separate group. Ectoprocts are still often called bryozoans. Sequence analysis places both entoprocts and ectoprocts among lophotrochozoan phyla.
Acanthocephalans are highly specialized parasites with a unique morphology and have doubtless been so for millions of years. Any ancestral or other related group that would shed a clue to phyletic relationships of Acanthocephala is probably long since extinct. Like cestodes, acanthocephalans have no digestive tract and must absorb all nutrients across their tegument, but the tegument of the two groups is quite different in structure. Also, acanthocephalans are pseudocoelomate and show eutely, as do nematodes, although here, too, structural and developmental differences are great.
Adaptive Radiation
Certainly the most impressive adaptive radiation in this group of phyla is shown by nematodes. They are by far the most numerous in terms of both individuals and species, and they have been able to adapt to almost every habitat available to animal life. Their basic pseudocoelomate body plan, with the cuticle, hydrostatic skeleton, and longitudinal muscles, has proved generalized and plastic enough to adapt to an enormous variety of physical conditions. Free-living lines gave rise to parasitic forms on at least several occasions, and virtually all potential hosts have been exploited. All types of life cycle occur: from simple and direct to complex, with intermediate hosts; from normal dioecious reproduction to parthenogenesis, hermaphroditism, and alternation of free-living and parasitic generations. A major factor contributing to evolutionary opportunism of nematodes has been their extraordinary capacity to survive suboptimal conditions, for example, developmental arrests in many free-living and animal parasitic species and ability to undergo cryptobiosis (survival in harsh conditions by assuming a very low metabolic rate) in many freeliving and plant parasitic species.




