Structural and Functional Adaptations of Fishes
Structural and
Functional
Adaptations
of Fishes
Locomotion in Water
To the human eye, some fishes appear capable of swimming at extremely high speeds. But our judgment is unconsciously tempered by our own experience that water is a highly resistant medium through which to move. Most fishes, such as a trout or a minnow, can swim maximally about 10 body lengths per second, obviously an impressive performance by human standards. Yet when these speeds are translated into kilometers per hour it means that a 30 cm (1 foot) trout can swim only about 10.4 km (6.5 miles) per hour. As a general rule, the larger the fish the faster it can swim.
The propulsive mechanism of a fish is its trunk and tail musculature. The axial, locomotory musculature is composed of zigzag bands, called myomeres. Muscle fibers in each myomere are relatively short and connect the tough connective tissue partitions that separate each myomere from the next. On the surface the myomeres take the shape of a W lying on its side (Figure 26-24) but internally the bands are complexly folded and nested so that the pull of each myomere extends over several vertebrae. This arrangement produces more power and finer control of movement since many myomeres are involved in bending a given segment of the body.
Understanding how fishes swim
can be approached by studying the
motion of a very flexible fish such as an
eel (Figure 26-25). The movement is
serpentine, not unlike that of a snake,
with waves of contraction moving
backward along the body by alternate
contraction of the myomeres on either
side. The anterior end of the body
bends less than the posterior end, so
that each undulation increases in amplitude
as it travels along the body. While
undulations move backward, the bending
of the body pushes laterally against
the water, producing a reactive force that is directed forward, but at an angle.
It can be analyzed as having two components: thrust, which is used to overcome
drag and propels the fish forward,
and lateral force, which tends
to make the fish’s head “yaw,” or deviate
from the course in the same direction
as the tail. This side-to-side head
movement is very obvious in a swimming
eel or shark, but many fishes have
a large, rigid head with enough surface
resistance to minimize yaw.
The movement of an eel is reasonably efficient at low speed, but its body shape generates too much frictional drag for rapid swimming. Fishes that swim rapidly, such as trout, are less flexible and limit body undulations mostly to the caudal region (Figure 26-25). Muscle force generated in the large anterior muscle mass is transferred through tendons to the relatively nonmuscular caudal peduncle and tail where thrust is generated. This form of swimming reaches its highest development in the tunas, whose bodies do not flex at all. Virtually all thrust is derived from powerful beats of the tail fin (Figure 26-26). Many fast oceanic fishes such as marlin, swordfish, amberjacks, and wahoo have sweptback tail fins shaped much like a sickle. Such fins are the aquatic counterpart of the high-aspect ratio wings of the swiftest birds.
Swimming is the most economical form of animal locomotion, largely because aquatic animals are almost perfectly supported by their medium and need expend little energy to overcome the force of gravity. If we compare the energy cost per kilogram of body weight of traveling 1 km by different forms of locomotion, we find swimming costs only 0.39 kcal (salmon) as compared with 1.45 kcal for flying (gull) and 5.43 for walking (ground squirrel). However, part of the unfinished business of biology is understanding how fish and aquatic mammals are able to move through the water while creating almost no turbulence. The secret lies in the way aquatic animals bend their bodies and fins (or flukes) to swim and in the friction-reducing properties of the body surface.
Neutral Buoyancy and the Swim Bladder
All fishes are slightly heavier than water because their skeletons and other tissues contain heavy elements that are present only in trace amounts in natural waters. To keep from sinking, sharks must always keep moving forward in the water. The asymmetrical (heterocercal) tail of a shark provides the necessary tail lift as it sweeps to and fro in the water, and the broad head and flat pectoral fins (Figure 26-8) act as angled planes to provide head lift. Sharks are also aided in buoyancy by having very large livers containing a special fatty hydrocarbon called squalene with a density of only 0.86. The liver thus acts like a large sack of buoyant oil that helps to compensate for the shark’s heavy body.
By far the most efficient flotation device is a gas-filled space. The swim bladder serves this purpose in the bony fishes (Figure 26-27). It arose from the paired lungs of the primitive Devonian bony fishes. Lungs were probably a ubiquitous feature of the Devonian freshwater bony fishes when, as we have seen, warm, swampy habitats would have made such an accessory respiratory structure advantageous. Swim bladders are present in most pelagic bony fishes but are absent in tunas, most abyssal fishes, and most bottom dwellers, such as flounders and sculpins.
By adjusting the volume of gas in the swim bladder, a fish can achieve neutral buoyancy and remain suspended indefinitely at any depth with no muscular effort. There are severe technical problems, however. If the fish descends to a greater depth, the swim bladder gas is compressed so that the fish becomes heavier and tends to sink. Gas must be added to the bladder to establish a new equilibrium buoyancy. If the fish swims upward, the gas in the bladder expands, making the fish lighter. Unless gas is removed, the fish will rise with ever-increasing speed while the bladder continues to expand.
Gas may be removed from the swim bladder in one of two ways. The more primitive phystostomous (Gr., phys, bladder, stoma, mouth) fishes (trout, for example) have a pneumatic duct that connects the swim bladder to the esophagus. These fishes may simply expel air out through the pneumatic duct. More advanced teleosts exhibit the physoclistous (Gr., phys, bladder, clist, closed) condition in which the pneumatic duct is lost in adults. In physoclistous fishes, gas must be secreted into the blood from the ovale, a vascularized area (Figure 26-27). Both types of fishes require gas to be secreted into the swim bladder from the blood, although a few shallow-water-inhabiting phystostomes may gulp air to fill their swim bladder.
Gas is secreted into the swim bladder at the highly specialized gas gland. The gas gland is supplied by a remarkable network of blood capillaries, called the rete mirabile (“marvelous net”) that functions as a countercurrent exchange system to trap gases, especially oxygen, and prevent their loss to the circulation (Figure 26-27)
The amazing effectiveness of this device is exemplified by a fish living at a depth of 2400 m (8000 feet). To keep the bladder inflated at that depth, the gas inside (mostly oxygen, but also variable amounts of nitrogen, carbon dioxide, argon, and even some carbon monoxide) must have a pressure exceeding 240 atmospheres, which is much greater than the pressure in a fully charged steel gas cylinder. Yet the oxygen pressure in the fish’s blood cannot exceed 0.2 atmosphere—equal to the oxygen pressure at the sea surface.
Physiologists who were at first baffled by the secretion mechanism now understand how it operates. In brief, the gas gland secretes lactic acid, which enters the blood, causing a localized high acidity in the rete mirabile that forces hemoglobin to release its load of oxygen. The capillaries in the rete are arranged so that the released oxygen accumulates in the rete, eventually reaching such a high pressure that the oxygen diffuses into the swim bladder. The final gas pressure attained in the swim bladder depends on the length of the rete capillaries; they are relatively short in fishes living near the surface, but are extremely long in deep-sea fishes.
Respiration
Fish gills are composed of thin filaments, each covered with a thin epidermal membrane that is folded repeatedly into platelike lamellae (Figure 26-28). These are richly supplied with blood vessels. The gills are located inside the pharyngeal cavity and are covered with a movable flap, the operculum. This arrangement provides excellent protection to the delicate gill filaments, streamlines the body, and makes possible a pumping system for moving water through the mouth, across the gills, and out the operculum. Instead of opercular flaps as in bony fishes, the elasmobranchs have a series of gill slits (Figure 26-8) out of which the water flows. In both elasmobranchs and bony fishes the branchial mechanism is arranged to pump water continuously and smoothly over the gills, although to an observer it appears that fish breathing is pulsatile. The flow of water is opposite to the direction of blood flow (countercurrent flow), the best arrangement for extracting the greatest possible amount of oxygen from the water. Some bony fishes can remove as much as 85% of the oxygen from water passing over their gills. Very active fishes, such as herring and mackerel, can obtain sufficient water for their high oxygen demands only by swimming forward continuously to force water into the open mouth and across the gills. This process is called ram ventilation. Such fish will be asphyxiated if placed in an aquarium that restricts free swimming movements, even if the water is saturated with oxygen.
A surprising number of fishes can live out of water for varying lengths of time by breathing air. Several devices are employed by different fishes. We already have described the lungs of the lungfishes, Polypterus, and the extinct rhipidistians. Freshwater eels often make overland excursions during rainy weather, using the skin as a major respiratory surface. The bowfin, Amia, has both gills and a lunglike swim bladder. At low temperatures it uses only its gills, but as the temperature and the fish’s activity increase, it breathes mostly air with its swim bladder. The electric eel, Electrophorus (Gr. e-lektron, something bright, + phoros, to bear), has degenerate gills and must supplement gill respiration by gulping air through its vascular mouth cavity. One of the best air breathers of all is the Indian climbing perch Anabas (Gr. anabaino-, to go up), which spends most of its time on land near the water’s edge, breathing air through special air chambers above muchreduced gills.
Osmotic Regulation
Fresh water is an extremely dilute medium with a salt concentration (0.001 to 0.005 gram moles per liter [M]) much below that of the blood of freshwater fishes (0.2 to 0.3 M). Water therefore tends to enter their bodies osmotically, and salt is lost by diffusion outward. Although the scaled and mucous-covered body surface is almost totally impermeable to water, water gain and salt loss do occur across thin membranes of the gills. Freshwater fishes are hyperosmotic regulators that have several defenses against these problems (Figure 26-29). First, the excess water is pumped out by the opisthonephric kidney, which is capable of forming very dilute urine. Second, special saltabsorbing cells located in the gill epithelium actively move salt ions, principally sodium and chloride, from the water to the blood. This, together with salt present in the fish’s food, replaces diffusive salt loss. These mechanisms are so efficient that a freshwater fish devotes only a small part of its total energy expenditure to keeping itself in osmotic balance.
Marine bony fishes are hypoosmotic regulators that encounter a completely different set of problems. Having a much lower blood salt concentration (0.3 to 0.4 M) than the seawater around them (about 1 M), they tend to lose water and gain salt. The marine teleost fish quite literally risks drying out, much like a desert mammal deprived of water. Again, marine bony fishes, like their freshwater counterparts, have evolved an appropriate set of defenses (Figure 26-29). To compensate for water loss, the marine teleost drinks seawater. Although this behavior obviously brings needed water into the body, it is unfortunately accompanied by a great deal of unneeded salt. Unwanted salt is disposed in two ways: (1) the major sea salt ions (sodium, chloride, and potassium) are carried by the blood to the gills where they are secreted outward by special salt-secretory cells; and (2) the remaining ions, mostly the divalent ions (magnesium, sulfate, and calcium), are left in the intestine and voided with the feces. However, a small but significant fraction of these residual divalent salts in the intestine, some 10% to 40% of the total, penetrates the intestinal mucosa and enters the bloodstream. These ions are excreted by the kidney. Unlike the freshwater fish kidney, which forms its urine by the usual filtrationresorption sequence typical of most vertebrate kidneys, the marine fish’s kidney excretes divalent ions by tubular secretion. Since very little if any filtrate is formed, the glomeruli have lost their importance and disappeared altogether in some marine teleosts. The pipefishes, and the goosefish shown in Figure 26-31, are examples of “aglomerular” marine fishes.
Feeding Behavior
For any fish, feeding is one of the main concerns of day-to-day living. Although many a luckless angler would swear otherwise, the fact is that a fish devotes more time and energy to eating, or searching for food to eat, than to anything else. Throughout the long evolution of fishes, there has been unrelenting selective pressure for those adaptations that enable a fish to come out on the better end of the eat-or-beeaten contest. Certainly the most farreaching single event was the evolution of jaws. Their possessors were freed from a largely passive filterfeeding existence and could adopt a predatory mode of life. Improved means of capturing larger prey demanded stronger muscles, more agile movement, better balance, and improved special senses. More than any other aspect of its life habit, feeding behavior shapes the fish.
Most fishes are carnivores that prey on a myriad of animal foods from zooplankton and insect larvae to large vertebrates. Some deep-sea fishes are capable of eating victims nearly twice their own size—an adaptation for life in a world where meals are necessarily infrequent. Most advanced ray-finned fishes cannot masticate their food as we can because doing so would block the current of water across the gills. Some, however, such as the wolf eel (Figure 26-30), have molarlike teeth in the jaws for crushing their prey, which may include hard-bodied crustaceans. Others that do grind their food use powerful pharyngeal teeth in the throat. Most carnivorous fish almost invariably swallow their prey whole, using sharppointed teeth in the jaws and on the roof of the mouth to seize their prey. The incompressibility of water makes the task even easier for many largemouthed predators. When the mouth is suddenly opened, a negative pressure is created that sweeps the victim inside (Figure 26-31)
A second group of fishes are herbivores that eat plants and algae. Although plant eaters are relatively few in number, they are crucial intermediates in the food chain, especially in freshwater rivers, lakes, and ponds that contain very little plankton.
Suspension-feeders that crop the abundant microorganisms of the sea form a third and diverse group of fishes ranging from fish larvae to basking sharks. However, the most characteristic group of plankton feeders are herringlike fishes (menhaden, herring, anchovies, capelin, pilchards, and others), mostly pelagic (open-sea dwellers) fishes that travel in large schools. Both phytoplankton and the smaller zooplankton are strained from the water with the sievelike gill rakers (Figure 26-28). Because plankton feeders are the most abundant of all marine fishes, they are important food for numerous larger but less abundant carnivores. Many freshwater fishes also depend on plankton for food.
A fourth group of fishes contains omnivores that feed on both plant and animal food. Finally there are scavengers that feed on organic debris (detritus) and parasites that suck the body fluids of other fishes.
Digestion in most fishes follows the vertebrate plan. Except in several fishes that lack stomachs altogether, the food proceeds from stomach to tubular intestine, which tends to be short in carnivores (Figure 26-15) but may be extremely long and coiled in herbivorous forms. In the herbivorous grass carp, for example, the intestine may be nine times the body length, an adaptation for the lengthy digestion required for plant carbohydrates. In carnivores, some protein digestion may be initiated in the acid medium of the stomach, but the principal function of the stomach is to store the often large and infrequent meals while awaiting their reception by the intestine.
Digestion and absorption proceed simultaneously in the intestine. A curious feature of ray-finned fishes, especially the teleosts, is the presence of numerous pyloric ceca (Figure 26-15) found in no other vertebrate group. Their primary function appears to be fat absorption, although all classes of digestive enzymes (protein-, carbohydrate-, and fat-splitting) are secreted there.
Migration
Eel
For centuries naturalists had been puzzled about the life history of the freshwater eel Anguilla (an-gwil´la) (L. eel), a common and commercially important species of coastal streams of the North Atlantic. Eels are catadromous (Gr. kata, down, + dromos, running), meaning that they spend most of their lives in fresh water but migrate to the sea to spawn. Each fall, large numbers of eels were seen swimming down the rivers toward the sea, but no adults ever returned. Each spring countless numbers of young eels, called “elvers” (Figure 26-32), each about the size of a wooden matchstick, appeared in the coastal rivers and began swimming upstream. Beyond the assumption that eels must spawn somewhere at sea, location of their breeding grounds was completely unknown.
The first clue was provided by two Italian scientists, Grassi and Calandruccio, who in 1896 reported that elvers were not larval eels but rather were relatively advanced juveniles. True larval eels, they discovered, were tiny, leafshaped, completely transparent creatures that bore absolutely no resemblance to an eel. They had been called leptocephali (Gr. leptos, slender, + kephale, head) by early naturalists, who never suspected their true identity. In 1905 Johann Schmidt, supported by the Danish government, began a systematic study of eel biology that he continued until his death in 1933. With cooperation of captains of commercial vessels plying the Atlantic, thousands of leptocephali were caught in different areas of the Atlantic with the plankton nets Schmidt supplied them. By noting where larvae in different stages of development were captured, Schmidt and his colleagues eventually reconstructed the spawning migrations.
When adult eels leave the coastal rivers of Europe and North America, they swim steadily and apparently at great depth for 1 to 2 months until they reach the Sargasso Sea, a vast area of warm oceanic water southeast of Bermuda (Figure 26-32). Here, at depths of 300 m or more, the eels spawn and die. The minute larvae then begin an incredible journey back to the coastal rivers of Europe. Drifting with the Gulf Stream and preyed on constantly by numerous predators, they reach the middle of the Atlantic after 2 years. By the end of the third year they arrive in the coastal waters of Europe where the leptocephali metamorphose into elvers, with an unmistakable eel-like body form (Figure 26-32). Here the males and females part company; males remain in the brackish waters of coastal rivers and estuaries while females continue up the rivers, often traveling hundreds of miles upstream. After 8 to 15 years of growth, the females, now 1 m or more long, return to the sea to join the smaller males; both return to the ancestral breeding grounds thousands of miles away to complete the life cycle.
Schmidt found that the American eel (Anguilla rostrata) could be distinguished from the European eel (A. vulgaris) because it had fewer vertebrae— an average of 107 in the American eel as compared with an average 114 in the European species. Since the American eel is much closer to the North American coastline, it requires only about 8 months to make the journey.
Homing Salmon
The life history of salmon is nearly as remarkable as that of the eel and certainly has received far more popular attention. Salmon are anadromous (Gr. anadromos, running upward); that is, they spend their adult lives at sea but return to fresh water to spawn. The Atlantic salmon (Salmo salar) (L. salmo, salmon, sal, salt) and the Pacific salmon (six species in the genus Oncorhynchus [on-ko-rink´us] [Gr. onkos, hook, + rhynchos, snout]) have this practice, but there are important differences among the seven species. The Atlantic salmon may make repeated upstream spawning runs. The six Pacific salmon species (king, sockeye, silver, humpback, chum, and Japanese masu) each make a single spawning run (Figure 26-33), after which they die.
The virtually infallible homing instinct of the Pacific species is legendary: after migrating downstream as a smolt, a sockeye salmon ranges many hundreds of miles over the Pacific for nearly 4 years, grows to 2 to 5 kg in weight, and then returns almost unerringly to spawn in the headwaters of its parent stream. Some straying does occur and is an important means of increasing gene flow and populating new streams.
Experiments by A. D. Hasler and others have shown that homing salmon are guided upstream by the characteristic odor of their parent stream. When the salmon finally reach the spawning beds of their parents (where they themselves were hatched), they spawn and die. The following spring, newly hatched fry transform into smolts before and during the downstream migration. At this time they are imprinted with the distinctive odor of the stream, which is apparently a mosaic of compounds released by the characteristic vegetation and soil in the watershed of the parent stream. They also seem to imprint on the odors of other streams they pass while migrating downriver and use these odors in reverse sequence as a map during the upriver migration as returning adults.
How do salmon find their way to the mouth of the coastal river from the trackless miles of the open ocean? Salmon move hundreds of miles away from the coast, much too far to be able to detect the odor of their parent stream. Experiments suggest that some migrating fish, like birds, can navigate by orienting to the position of the sun. However, migrant salmon can navigate on cloudy days and at night, indicating that sun navigation, if used at all, cannot be the salmon’s only navigational cue. Fish also (again, like birds) appear able to detect and navigate to the earth’s magnetic field. Finally, fishery biologists concede that salmon may not require precise navigational abilities at all, but instead may use ocean currents, temperature gradients, and food availability to reach the general coastal area where “their” river is located. From this point, they would navigate by their imprinted odor map, making correct turns at each stream junction until they reach their natal stream.
Reproduction and Growth
In a group as diverse as the fishes, it is no surprise to find extraordinary variations on the basic theme of sexual reproduction. Most fishes favor a simple theme: they are dioecious, with external fertilization and external development of the eggs and embryos (oviparity). However, as tropical fish enthusiasts are well aware, the ever-popular ovoviviparous guppies and mollies of home aquaria bear their young alive after development in the ovarian cavity of the mother (Figure 26-34). As described earlier in this section, some viviparous sharks develop a kind of placental attachment through which the young are nourished during gestation.
Let us return to the much more common oviparous mode of reproduction. Many marine fishes are extraordinarily profligate egg producers. Males and females come together in great schools and release vast numbers of gametes into the water to drift with the current. Large female cod may release 4 to 6 million eggs at a single spawning. Less than one in a million will survive the numerous perils of the ocean to reach reproductive maturity.
Unlike the minute, buoyant, transparent
eggs of pelagic marine teleosts,
those of many near-shore bottomdwelling
(benthic) species are larger,
typically yolky, nonbuoyant, and adhesive.
Some bury their eggs, many attach
them to vegetation, some deposit them
in nests, and some even incubate them
in their mouths (Figure 26-35). Many
benthic spawners guard their eggs.
Intruders expecting an easy meal of
eggs may be met with a vivid and often
belligerent display by the guard, which
is almost always the male.
Freshwater fishes almost invariably produce nonbuoyant eggs. Those, such as perch, that provide no parental care simply scatter their myriads of eggs among weeds or along the bottom. Freshwater fishes that do provide some form of egg care, such as bullhead catfishes and some darters, produce fewer, larger eggs that enjoy a better chance for survival.
Elaborate preliminaries to mating are the rule for freshwater fishes. The female Pacific salmon, for example, performs a ritualized mating “dance” with her breeding partner after arriving at the spawning bed in a fast-flowing, gravel-bottomed stream (Figure 26-36). She then turns on her side and scoops out a nest with her tail. As the eggs are laid by the female, they are fertilized by the male (Figure 26-36). After the female covers the eggs with gravel, the exhausted fish dies and drifts downstream.
Soon after the egg of an oviparous species is laid and fertilized, it takes up water and the outer layer hardens. Cleavage follows, and the blastoderm forms, sitting astride a relatively enormous yolk mass. Soon the yolk mass is enclosed by the developing blastoderm, which then begins to assume a fishlike shape. The fish hatches as a larva carrying a semitransparent sac of yolk, which provides its food supply until the mouth and digestive tract have developed. The larva then begins searching for its own food. After a period of growth the larva undergoes a metamorphosis, especially dramatic in many marine species such as the freshwater eel described previously (Figure 26-32). Body shape is refashioned, fin and color patterns change, and the animal becomes a juvenile bearing the unmistakable definitive body form of its species.
Growth is temperature dependent.
Consequently, fish living in
temperate regions grow rapidly in
summer when temperatures are high
and food is abundant but nearly stop
growing in winter. Annual rings in
the scales, otoliths, and other bony
parts reflect this seasonal growth
(Figure 26-37), a distinctive record of
convenience to fishery biologists who
wish to determine a fish’s age. Unlike
birds and mammals, which stop
growing after reaching adult size,
most fishes after attaining reproductive
maturity continue to grow for as
long as they live. This may be a selective
advantage, since the larger the
fish, the more gametes it produces
and the greater its contribution to
future generations.
Classification of Living Fishes
The following Linnaean classification of major fish taxa follows that of Nelson (1994). The probable relationships of these traditional groupings together with the major extinct groups of fishes are shown in a cladogram in Figure 26-2. Other schemes of classification have been proposed. Because of the difficulty of determining relationships among the numerous living and fossil species, we can appreciate why fish classification has undergone, and will continue to undergo, continuous revision.
Phylum Chordata
Locomotion in Water
To the human eye, some fishes appear capable of swimming at extremely high speeds. But our judgment is unconsciously tempered by our own experience that water is a highly resistant medium through which to move. Most fishes, such as a trout or a minnow, can swim maximally about 10 body lengths per second, obviously an impressive performance by human standards. Yet when these speeds are translated into kilometers per hour it means that a 30 cm (1 foot) trout can swim only about 10.4 km (6.5 miles) per hour. As a general rule, the larger the fish the faster it can swim.
The propulsive mechanism of a fish is its trunk and tail musculature. The axial, locomotory musculature is composed of zigzag bands, called myomeres. Muscle fibers in each myomere are relatively short and connect the tough connective tissue partitions that separate each myomere from the next. On the surface the myomeres take the shape of a W lying on its side (Figure 26-24) but internally the bands are complexly folded and nested so that the pull of each myomere extends over several vertebrae. This arrangement produces more power and finer control of movement since many myomeres are involved in bending a given segment of the body.
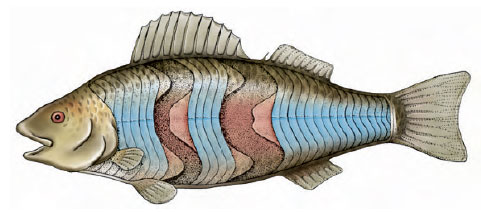 |
| Figure 26-24 Trunk musculature of a teleost fish, partly dissected to show internal arrangement of the muscle bands (myomeres). The myomeres are folded into a complex, nested grouping, an arrangement that favors stronger and more controlled swimming. |
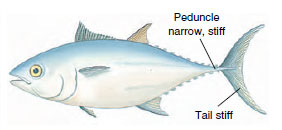 |
| Figure 26-26 Bluefin tuna, showing adaptations for fast swimming. Powerful trunk muscles pull on the slender tail stalk. Since the body does not bend, all of the thrust comes from beats of the stiff sickle-shaped tail. |
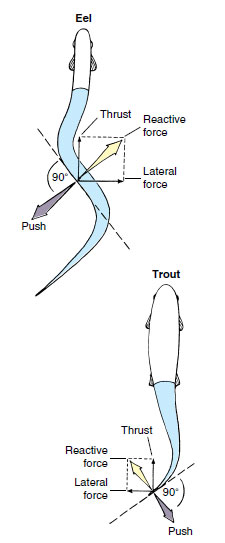 |
| Figure 26-25 Movements of swimming fishes, showing the forces developed by an eel-shaped and spindleshaped fish. |
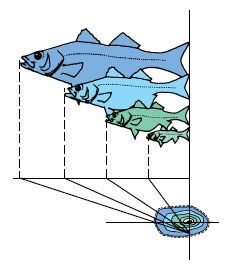
The movement of an eel is reasonably efficient at low speed, but its body shape generates too much frictional drag for rapid swimming. Fishes that swim rapidly, such as trout, are less flexible and limit body undulations mostly to the caudal region (Figure 26-25). Muscle force generated in the large anterior muscle mass is transferred through tendons to the relatively nonmuscular caudal peduncle and tail where thrust is generated. This form of swimming reaches its highest development in the tunas, whose bodies do not flex at all. Virtually all thrust is derived from powerful beats of the tail fin (Figure 26-26). Many fast oceanic fishes such as marlin, swordfish, amberjacks, and wahoo have sweptback tail fins shaped much like a sickle. Such fins are the aquatic counterpart of the high-aspect ratio wings of the swiftest birds.
Swimming is the most economical form of animal locomotion, largely because aquatic animals are almost perfectly supported by their medium and need expend little energy to overcome the force of gravity. If we compare the energy cost per kilogram of body weight of traveling 1 km by different forms of locomotion, we find swimming costs only 0.39 kcal (salmon) as compared with 1.45 kcal for flying (gull) and 5.43 for walking (ground squirrel). However, part of the unfinished business of biology is understanding how fish and aquatic mammals are able to move through the water while creating almost no turbulence. The secret lies in the way aquatic animals bend their bodies and fins (or flukes) to swim and in the friction-reducing properties of the body surface.
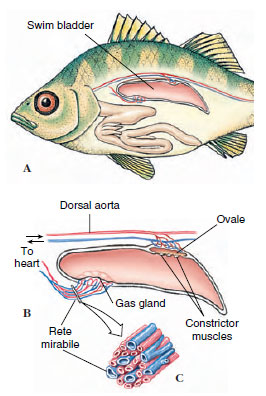 |
| Figure 26-27 A, Swim bladder of a teleost fish. The swim bladder lies in the coelom just beneath the vertebral column. B, Gas is secreted into the swim bladder by the gas gland. Gas from the blood is moved into the gas gland by the rete mirabile, a complex array of tightly- packed capillaries that act as a countercurrent multiplier to build up the oxygen concentration. The arrangement of venous and arterial capillaries in the rete is shown in C. To release gas during ascent, a muscular valve opens, allowing gas to enter the ovale from which the gas is removed by the circulation. |
Neutral Buoyancy and the Swim Bladder
All fishes are slightly heavier than water because their skeletons and other tissues contain heavy elements that are present only in trace amounts in natural waters. To keep from sinking, sharks must always keep moving forward in the water. The asymmetrical (heterocercal) tail of a shark provides the necessary tail lift as it sweeps to and fro in the water, and the broad head and flat pectoral fins (Figure 26-8) act as angled planes to provide head lift. Sharks are also aided in buoyancy by having very large livers containing a special fatty hydrocarbon called squalene with a density of only 0.86. The liver thus acts like a large sack of buoyant oil that helps to compensate for the shark’s heavy body.
By far the most efficient flotation device is a gas-filled space. The swim bladder serves this purpose in the bony fishes (Figure 26-27). It arose from the paired lungs of the primitive Devonian bony fishes. Lungs were probably a ubiquitous feature of the Devonian freshwater bony fishes when, as we have seen, warm, swampy habitats would have made such an accessory respiratory structure advantageous. Swim bladders are present in most pelagic bony fishes but are absent in tunas, most abyssal fishes, and most bottom dwellers, such as flounders and sculpins.
By adjusting the volume of gas in the swim bladder, a fish can achieve neutral buoyancy and remain suspended indefinitely at any depth with no muscular effort. There are severe technical problems, however. If the fish descends to a greater depth, the swim bladder gas is compressed so that the fish becomes heavier and tends to sink. Gas must be added to the bladder to establish a new equilibrium buoyancy. If the fish swims upward, the gas in the bladder expands, making the fish lighter. Unless gas is removed, the fish will rise with ever-increasing speed while the bladder continues to expand.
Gas may be removed from the swim bladder in one of two ways. The more primitive phystostomous (Gr., phys, bladder, stoma, mouth) fishes (trout, for example) have a pneumatic duct that connects the swim bladder to the esophagus. These fishes may simply expel air out through the pneumatic duct. More advanced teleosts exhibit the physoclistous (Gr., phys, bladder, clist, closed) condition in which the pneumatic duct is lost in adults. In physoclistous fishes, gas must be secreted into the blood from the ovale, a vascularized area (Figure 26-27). Both types of fishes require gas to be secreted into the swim bladder from the blood, although a few shallow-water-inhabiting phystostomes may gulp air to fill their swim bladder.
Gas is secreted into the swim bladder at the highly specialized gas gland. The gas gland is supplied by a remarkable network of blood capillaries, called the rete mirabile (“marvelous net”) that functions as a countercurrent exchange system to trap gases, especially oxygen, and prevent their loss to the circulation (Figure 26-27)
The amazing effectiveness of this device is exemplified by a fish living at a depth of 2400 m (8000 feet). To keep the bladder inflated at that depth, the gas inside (mostly oxygen, but also variable amounts of nitrogen, carbon dioxide, argon, and even some carbon monoxide) must have a pressure exceeding 240 atmospheres, which is much greater than the pressure in a fully charged steel gas cylinder. Yet the oxygen pressure in the fish’s blood cannot exceed 0.2 atmosphere—equal to the oxygen pressure at the sea surface.
Physiologists who were at first baffled by the secretion mechanism now understand how it operates. In brief, the gas gland secretes lactic acid, which enters the blood, causing a localized high acidity in the rete mirabile that forces hemoglobin to release its load of oxygen. The capillaries in the rete are arranged so that the released oxygen accumulates in the rete, eventually reaching such a high pressure that the oxygen diffuses into the swim bladder. The final gas pressure attained in the swim bladder depends on the length of the rete capillaries; they are relatively short in fishes living near the surface, but are extremely long in deep-sea fishes.
Respiration
Fish gills are composed of thin filaments, each covered with a thin epidermal membrane that is folded repeatedly into platelike lamellae (Figure 26-28). These are richly supplied with blood vessels. The gills are located inside the pharyngeal cavity and are covered with a movable flap, the operculum. This arrangement provides excellent protection to the delicate gill filaments, streamlines the body, and makes possible a pumping system for moving water through the mouth, across the gills, and out the operculum. Instead of opercular flaps as in bony fishes, the elasmobranchs have a series of gill slits (Figure 26-8) out of which the water flows. In both elasmobranchs and bony fishes the branchial mechanism is arranged to pump water continuously and smoothly over the gills, although to an observer it appears that fish breathing is pulsatile. The flow of water is opposite to the direction of blood flow (countercurrent flow), the best arrangement for extracting the greatest possible amount of oxygen from the water. Some bony fishes can remove as much as 85% of the oxygen from water passing over their gills. Very active fishes, such as herring and mackerel, can obtain sufficient water for their high oxygen demands only by swimming forward continuously to force water into the open mouth and across the gills. This process is called ram ventilation. Such fish will be asphyxiated if placed in an aquarium that restricts free swimming movements, even if the water is saturated with oxygen.
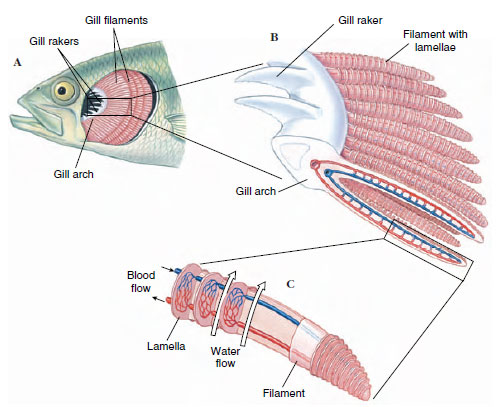 |
| Figure 26-28 Gills of fish. Bony, protective flap covering the gills (operculum) has been removed, A, to reveal branchial chamber containing the gills. There are four gill arches on each side, each bearing numerous filaments. A portion of gill arch (B) shows gill rakers that project forward to strain out food and debris, and gill filaments that project to the rear. A single gill filament (C) is dissected to show the blood capillaries within the platelike lamellae. Direction of water flow (large arrows) is opposite the direction of blood flow. |
A surprising number of fishes can live out of water for varying lengths of time by breathing air. Several devices are employed by different fishes. We already have described the lungs of the lungfishes, Polypterus, and the extinct rhipidistians. Freshwater eels often make overland excursions during rainy weather, using the skin as a major respiratory surface. The bowfin, Amia, has both gills and a lunglike swim bladder. At low temperatures it uses only its gills, but as the temperature and the fish’s activity increase, it breathes mostly air with its swim bladder. The electric eel, Electrophorus (Gr. e-lektron, something bright, + phoros, to bear), has degenerate gills and must supplement gill respiration by gulping air through its vascular mouth cavity. One of the best air breathers of all is the Indian climbing perch Anabas (Gr. anabaino-, to go up), which spends most of its time on land near the water’s edge, breathing air through special air chambers above muchreduced gills.
Osmotic Regulation
Fresh water is an extremely dilute medium with a salt concentration (0.001 to 0.005 gram moles per liter [M]) much below that of the blood of freshwater fishes (0.2 to 0.3 M). Water therefore tends to enter their bodies osmotically, and salt is lost by diffusion outward. Although the scaled and mucous-covered body surface is almost totally impermeable to water, water gain and salt loss do occur across thin membranes of the gills. Freshwater fishes are hyperosmotic regulators that have several defenses against these problems (Figure 26-29). First, the excess water is pumped out by the opisthonephric kidney, which is capable of forming very dilute urine. Second, special saltabsorbing cells located in the gill epithelium actively move salt ions, principally sodium and chloride, from the water to the blood. This, together with salt present in the fish’s food, replaces diffusive salt loss. These mechanisms are so efficient that a freshwater fish devotes only a small part of its total energy expenditure to keeping itself in osmotic balance.
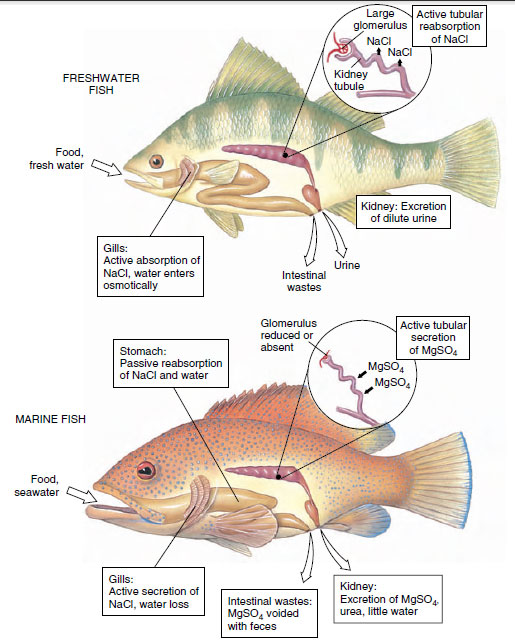 |
| Figure 26-29 Osmotic regulation in freshwater and marine bony fishes. A freshwater fish maintains osmotic and ionic balance in its dilute environment by actively absorbing sodium chloride across the gills (some salt is gained with food). To flush out excess that constantly enters the body, the glomerular kidney produces a dilute urine by reabsorbing sodium chloride. A marine fish must drink seawater to replace water lost osmotically to its salty environment. Sodium chloride and water are absorbed from the stomach. Excess sodium chloride is actively transported outward by the gills. Divalent sea salts, mostly magnesium sulfate, are eliminated with feces and secreted by the tubular kidney. |
Marine bony fishes are hypoosmotic regulators that encounter a completely different set of problems. Having a much lower blood salt concentration (0.3 to 0.4 M) than the seawater around them (about 1 M), they tend to lose water and gain salt. The marine teleost fish quite literally risks drying out, much like a desert mammal deprived of water. Again, marine bony fishes, like their freshwater counterparts, have evolved an appropriate set of defenses (Figure 26-29). To compensate for water loss, the marine teleost drinks seawater. Although this behavior obviously brings needed water into the body, it is unfortunately accompanied by a great deal of unneeded salt. Unwanted salt is disposed in two ways: (1) the major sea salt ions (sodium, chloride, and potassium) are carried by the blood to the gills where they are secreted outward by special salt-secretory cells; and (2) the remaining ions, mostly the divalent ions (magnesium, sulfate, and calcium), are left in the intestine and voided with the feces. However, a small but significant fraction of these residual divalent salts in the intestine, some 10% to 40% of the total, penetrates the intestinal mucosa and enters the bloodstream. These ions are excreted by the kidney. Unlike the freshwater fish kidney, which forms its urine by the usual filtrationresorption sequence typical of most vertebrate kidneys, the marine fish’s kidney excretes divalent ions by tubular secretion. Since very little if any filtrate is formed, the glomeruli have lost their importance and disappeared altogether in some marine teleosts. The pipefishes, and the goosefish shown in Figure 26-31, are examples of “aglomerular” marine fishes.
Feeding Behavior
For any fish, feeding is one of the main concerns of day-to-day living. Although many a luckless angler would swear otherwise, the fact is that a fish devotes more time and energy to eating, or searching for food to eat, than to anything else. Throughout the long evolution of fishes, there has been unrelenting selective pressure for those adaptations that enable a fish to come out on the better end of the eat-or-beeaten contest. Certainly the most farreaching single event was the evolution of jaws. Their possessors were freed from a largely passive filterfeeding existence and could adopt a predatory mode of life. Improved means of capturing larger prey demanded stronger muscles, more agile movement, better balance, and improved special senses. More than any other aspect of its life habit, feeding behavior shapes the fish.
Most fishes are carnivores that prey on a myriad of animal foods from zooplankton and insect larvae to large vertebrates. Some deep-sea fishes are capable of eating victims nearly twice their own size—an adaptation for life in a world where meals are necessarily infrequent. Most advanced ray-finned fishes cannot masticate their food as we can because doing so would block the current of water across the gills. Some, however, such as the wolf eel (Figure 26-30), have molarlike teeth in the jaws for crushing their prey, which may include hard-bodied crustaceans. Others that do grind their food use powerful pharyngeal teeth in the throat. Most carnivorous fish almost invariably swallow their prey whole, using sharppointed teeth in the jaws and on the roof of the mouth to seize their prey. The incompressibility of water makes the task even easier for many largemouthed predators. When the mouth is suddenly opened, a negative pressure is created that sweeps the victim inside (Figure 26-31)
 |
 |
|
| Figure 26-30 Wolf eel, Anarrhichthys ocellatus, feeding on a sea cucumber it has captured and pulled to the opening of its den. |
Figure 26-31 Goosefish Lophius piscatorius awaits its meal. Above its head swings a modified dorsal fin spine ending in a fleshy tentacle that contracts and expands in a convincing wormlike manner. When a fish approaches the alluring bait, the huge mouth opens suddenly, creating a strong inward current that sweeps the prey inside. In a split second all is over. |
A second group of fishes are herbivores that eat plants and algae. Although plant eaters are relatively few in number, they are crucial intermediates in the food chain, especially in freshwater rivers, lakes, and ponds that contain very little plankton.
Suspension-feeders that crop the abundant microorganisms of the sea form a third and diverse group of fishes ranging from fish larvae to basking sharks. However, the most characteristic group of plankton feeders are herringlike fishes (menhaden, herring, anchovies, capelin, pilchards, and others), mostly pelagic (open-sea dwellers) fishes that travel in large schools. Both phytoplankton and the smaller zooplankton are strained from the water with the sievelike gill rakers (Figure 26-28). Because plankton feeders are the most abundant of all marine fishes, they are important food for numerous larger but less abundant carnivores. Many freshwater fishes also depend on plankton for food.
A fourth group of fishes contains omnivores that feed on both plant and animal food. Finally there are scavengers that feed on organic debris (detritus) and parasites that suck the body fluids of other fishes.
Digestion in most fishes follows the vertebrate plan. Except in several fishes that lack stomachs altogether, the food proceeds from stomach to tubular intestine, which tends to be short in carnivores (Figure 26-15) but may be extremely long and coiled in herbivorous forms. In the herbivorous grass carp, for example, the intestine may be nine times the body length, an adaptation for the lengthy digestion required for plant carbohydrates. In carnivores, some protein digestion may be initiated in the acid medium of the stomach, but the principal function of the stomach is to store the often large and infrequent meals while awaiting their reception by the intestine.
Digestion and absorption proceed simultaneously in the intestine. A curious feature of ray-finned fishes, especially the teleosts, is the presence of numerous pyloric ceca (Figure 26-15) found in no other vertebrate group. Their primary function appears to be fat absorption, although all classes of digestive enzymes (protein-, carbohydrate-, and fat-splitting) are secreted there.
Migration
Eel
For centuries naturalists had been puzzled about the life history of the freshwater eel Anguilla (an-gwil´la) (L. eel), a common and commercially important species of coastal streams of the North Atlantic. Eels are catadromous (Gr. kata, down, + dromos, running), meaning that they spend most of their lives in fresh water but migrate to the sea to spawn. Each fall, large numbers of eels were seen swimming down the rivers toward the sea, but no adults ever returned. Each spring countless numbers of young eels, called “elvers” (Figure 26-32), each about the size of a wooden matchstick, appeared in the coastal rivers and began swimming upstream. Beyond the assumption that eels must spawn somewhere at sea, location of their breeding grounds was completely unknown.
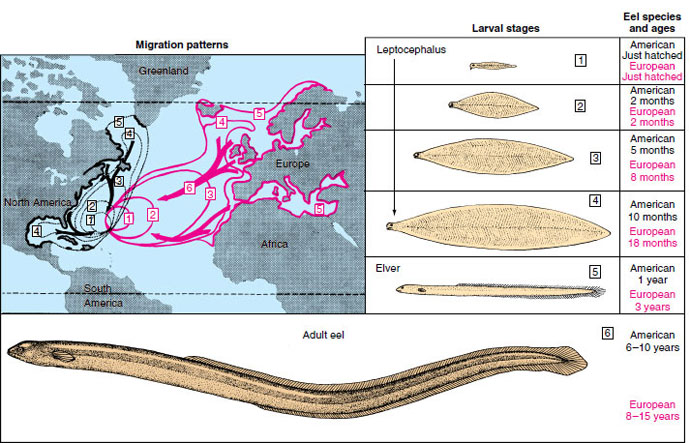 |
| Figure 26-32 Life histories of the European eel, Anguilla anguilla, and American eel, Anguilla rostrata. Migration patterns of European species are shown in pink. Migration patterns of American species are shown in black. Boxed numbers refer to stages of development. Note that the American eel completes its larval metamorphosis and sea journey in one year. It requires nearly three years for the European eel to complete its much longer journey. |
The first clue was provided by two Italian scientists, Grassi and Calandruccio, who in 1896 reported that elvers were not larval eels but rather were relatively advanced juveniles. True larval eels, they discovered, were tiny, leafshaped, completely transparent creatures that bore absolutely no resemblance to an eel. They had been called leptocephali (Gr. leptos, slender, + kephale, head) by early naturalists, who never suspected their true identity. In 1905 Johann Schmidt, supported by the Danish government, began a systematic study of eel biology that he continued until his death in 1933. With cooperation of captains of commercial vessels plying the Atlantic, thousands of leptocephali were caught in different areas of the Atlantic with the plankton nets Schmidt supplied them. By noting where larvae in different stages of development were captured, Schmidt and his colleagues eventually reconstructed the spawning migrations.
When adult eels leave the coastal rivers of Europe and North America, they swim steadily and apparently at great depth for 1 to 2 months until they reach the Sargasso Sea, a vast area of warm oceanic water southeast of Bermuda (Figure 26-32). Here, at depths of 300 m or more, the eels spawn and die. The minute larvae then begin an incredible journey back to the coastal rivers of Europe. Drifting with the Gulf Stream and preyed on constantly by numerous predators, they reach the middle of the Atlantic after 2 years. By the end of the third year they arrive in the coastal waters of Europe where the leptocephali metamorphose into elvers, with an unmistakable eel-like body form (Figure 26-32). Here the males and females part company; males remain in the brackish waters of coastal rivers and estuaries while females continue up the rivers, often traveling hundreds of miles upstream. After 8 to 15 years of growth, the females, now 1 m or more long, return to the sea to join the smaller males; both return to the ancestral breeding grounds thousands of miles away to complete the life cycle.
Schmidt found that the American eel (Anguilla rostrata) could be distinguished from the European eel (A. vulgaris) because it had fewer vertebrae— an average of 107 in the American eel as compared with an average 114 in the European species. Since the American eel is much closer to the North American coastline, it requires only about 8 months to make the journey.
 |
| Figure 26-33 Migrating Pacific sockeye salmon (Oncorhynchus nerka). |
Homing Salmon
The life history of salmon is nearly as remarkable as that of the eel and certainly has received far more popular attention. Salmon are anadromous (Gr. anadromos, running upward); that is, they spend their adult lives at sea but return to fresh water to spawn. The Atlantic salmon (Salmo salar) (L. salmo, salmon, sal, salt) and the Pacific salmon (six species in the genus Oncorhynchus [on-ko-rink´us] [Gr. onkos, hook, + rhynchos, snout]) have this practice, but there are important differences among the seven species. The Atlantic salmon may make repeated upstream spawning runs. The six Pacific salmon species (king, sockeye, silver, humpback, chum, and Japanese masu) each make a single spawning run (Figure 26-33), after which they die.
The virtually infallible homing instinct of the Pacific species is legendary: after migrating downstream as a smolt, a sockeye salmon ranges many hundreds of miles over the Pacific for nearly 4 years, grows to 2 to 5 kg in weight, and then returns almost unerringly to spawn in the headwaters of its parent stream. Some straying does occur and is an important means of increasing gene flow and populating new streams.
Experiments by A. D. Hasler and others have shown that homing salmon are guided upstream by the characteristic odor of their parent stream. When the salmon finally reach the spawning beds of their parents (where they themselves were hatched), they spawn and die. The following spring, newly hatched fry transform into smolts before and during the downstream migration. At this time they are imprinted with the distinctive odor of the stream, which is apparently a mosaic of compounds released by the characteristic vegetation and soil in the watershed of the parent stream. They also seem to imprint on the odors of other streams they pass while migrating downriver and use these odors in reverse sequence as a map during the upriver migration as returning adults.
How do salmon find their way to the mouth of the coastal river from the trackless miles of the open ocean? Salmon move hundreds of miles away from the coast, much too far to be able to detect the odor of their parent stream. Experiments suggest that some migrating fish, like birds, can navigate by orienting to the position of the sun. However, migrant salmon can navigate on cloudy days and at night, indicating that sun navigation, if used at all, cannot be the salmon’s only navigational cue. Fish also (again, like birds) appear able to detect and navigate to the earth’s magnetic field. Finally, fishery biologists concede that salmon may not require precise navigational abilities at all, but instead may use ocean currents, temperature gradients, and food availability to reach the general coastal area where “their” river is located. From this point, they would navigate by their imprinted odor map, making correct turns at each stream junction until they reach their natal stream.
 |
| Figure 26-34 Rainbow surfperch Hypsurus caryi giving birth. All of the West Coast surfperches (family Embiotocidae) are ovoviviparous. |
Reproduction and Growth
In a group as diverse as the fishes, it is no surprise to find extraordinary variations on the basic theme of sexual reproduction. Most fishes favor a simple theme: they are dioecious, with external fertilization and external development of the eggs and embryos (oviparity). However, as tropical fish enthusiasts are well aware, the ever-popular ovoviviparous guppies and mollies of home aquaria bear their young alive after development in the ovarian cavity of the mother (Figure 26-34). As described earlier in this section, some viviparous sharks develop a kind of placental attachment through which the young are nourished during gestation.
Let us return to the much more common oviparous mode of reproduction. Many marine fishes are extraordinarily profligate egg producers. Males and females come together in great schools and release vast numbers of gametes into the water to drift with the current. Large female cod may release 4 to 6 million eggs at a single spawning. Less than one in a million will survive the numerous perils of the ocean to reach reproductive maturity.
 |
| Figure 26-35 Male banded jawfish Opistognathus macrognathus orally brooding its eggs. The male retrieves the female’s spawn and incubates the eggs until they hatch. During brief periods when the jawfish is feeding, the eggs are left in the burrow. |
Freshwater fishes almost invariably produce nonbuoyant eggs. Those, such as perch, that provide no parental care simply scatter their myriads of eggs among weeds or along the bottom. Freshwater fishes that do provide some form of egg care, such as bullhead catfishes and some darters, produce fewer, larger eggs that enjoy a better chance for survival.
Elaborate preliminaries to mating are the rule for freshwater fishes. The female Pacific salmon, for example, performs a ritualized mating “dance” with her breeding partner after arriving at the spawning bed in a fast-flowing, gravel-bottomed stream (Figure 26-36). She then turns on her side and scoops out a nest with her tail. As the eggs are laid by the female, they are fertilized by the male (Figure 26-36). After the female covers the eggs with gravel, the exhausted fish dies and drifts downstream.
Soon after the egg of an oviparous species is laid and fertilized, it takes up water and the outer layer hardens. Cleavage follows, and the blastoderm forms, sitting astride a relatively enormous yolk mass. Soon the yolk mass is enclosed by the developing blastoderm, which then begins to assume a fishlike shape. The fish hatches as a larva carrying a semitransparent sac of yolk, which provides its food supply until the mouth and digestive tract have developed. The larva then begins searching for its own food. After a period of growth the larva undergoes a metamorphosis, especially dramatic in many marine species such as the freshwater eel described previously (Figure 26-32). Body shape is refashioned, fin and color patterns change, and the animal becomes a juvenile bearing the unmistakable definitive body form of its species.
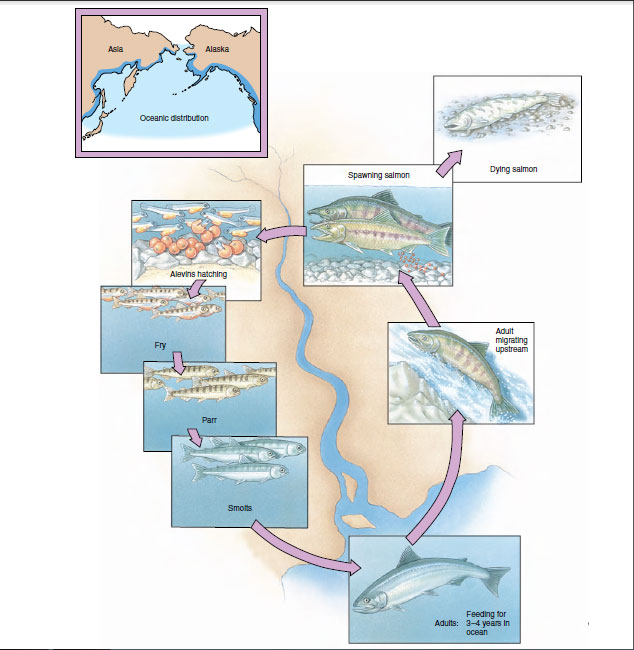 |
| Figure 26-36 Spawning Pacific salmon and development of the eggs and young. |
 |
| Figure 26-37 Scale growth. Fish scales disclose seasonal changes in growth rate. Growth is interrupted during winter, producing year marks (annuli). Each year’s increment in scale growth is a ratio to the annual increase in body length. Otoliths (ear stones) and certain bones can also be used in some species to determine age and growth rate. |
Classification of Living Fishes
The following Linnaean classification of major fish taxa follows that of Nelson (1994). The probable relationships of these traditional groupings together with the major extinct groups of fishes are shown in a cladogram in Figure 26-2. Other schemes of classification have been proposed. Because of the difficulty of determining relationships among the numerous living and fossil species, we can appreciate why fish classification has undergone, and will continue to undergo, continuous revision.
Phylum Chordata
-
Subphylum Vertebrata (Craniata)
-
Superclass Agnatha (ag'na-tha)
(Gr. a, not + gnathos, jaw). No
jaws; cartilaginous skeleton; paired
limbs absent; one or two semicircular
canals; notochord persistent.
Not a monophyletic taxon.
-
Class Myxini (mik-sy'ny) (Gr. myxa, slime): hagfishes. Four
pairs of tentacles around
mouth; nasal sac with duct to
pharynx; 5 to 15 pairs of gill
pouches, accessory hearts and
slime glands present; poorly
developed eyes. Examples. Myxine, Bdellostoma;
43 species, marine.
Class Cephalaspidomorphi (sef-a-lass'pe-do-morf'e) (Gr. kephalƒ, head, + aspidos, shield, + morphƒ, form): lampreys. Buccal funnel with keratinized teeth; nasal sac not connected to mouth; seven pairs of gill pouches; well-developed eyes. Examples: Petromyzon, Ichthyomyzon, Lampetra; 41 species, freshwater and anadromous.
-
Class Chondrichthyes (kondrik
'thee-eez) (Gr. chondros, cartilage + ichthys, fish): cartilaginous fishes. Cartilaginous
skeleton; teeth not fused to jaws
and usually replaced; no swim
bladder; intestine with spiral
valve; claspers present in males.
-
Subclass Elasmobranchii (e-laz'mo-bran'kee'i) (Gr. elasmos, plated, + branchia,
gills): sharks, skates, and
rays. Placoid scales or derivatives
(scutes and spines)
usually present; five to seven
gill arches and gills in separate
clefts along pharynx;
upper jaw not fused to cranium.
Examples: Squalus,
Raja, Charcarodon, Sphyrna.
About 815 species,
mostly marine.
Subclass Holocephali (hol'o-sef'a-li) (Gr. holos, entire, + kephalƒ, head): chimaeras, ratfishes. Scales absent; four gill slits covered by operculum; jaws with tooth plates; accessory clasping organ (tentaculum) in males; upper jaw fused to cranium. Examples: Chimaera, Hydrolagus; 31 species, marine.
-
Subclass Chrondrostei (kon-dros'tee-i) (Gr. chondros, cartilage, + osteon,
bone): bichirs, paddlefishes,
sturgeons. Skeleton
primarily cartilage; caudal fin
heterocercal; scales ganoid, if
present; spiral valve present;
spiracle usually present; more
fin rays than ray supports.
Examples: Polypterus, Polyodon,
Acipenser. 34 species,
freshwater and anadromous.
Subclass Neopterygii (nee'op-te-rij'ee-i) (Gr. neo, new, + pteryx, fin, wing): gars, bowfin, teleosts. Skeleton primarily bone; caudal fin usually homocercal; scales cycloid, ctenoid, absent, or rarely, ganoid. Fin ray number equal to their supports in dorsal and anal fins. Examples: Amia, Lepisosteus, Anguilla, Oncorhynchus, Perca. About 23,600 species, nearly all aquatic habitats.




