Forms of Nitrogen in Soils
Forms of Nitrogen in SoilsThe total nitrogen of the Earth is about 1.67 x 1023 g (89,90). Stevenson (89,90) reported that about 98% of the nitrogen of the Earth is in the lithosphere (rocks, soil, coal, sediments, core, sea bottom). About 2% of the nitrogen is in the atmosphere, with the portions in the hydrosphere and biosphere being insignificant relative to that in the lithosphere and atmosphere. Most of the nitrogen of the Earth, including the nitrogen in the rocks and in the atmosphere, is not available for plant nutrition. The nitrogen in soils, lakes, streams, sea bottoms, and living organisms is only about 0.02% of the total nitrogen of the Earth (89,90). Plants obtain most of their nitrogen nutrition from the soil. The nitrogen in the soil is about 2.22 x 1017 g, most of which is in soil organic matter and which is a negligible component of the total nitrogen content of the world (89,90).
Living organisms (biosphere) contain about 2.8 x 1017 g of nitrogen. The nitrogen of living organisms and of the soil is in a constant state of flux, with some forms of nitrogen being readily transformed in this group and some forms being inactive over a long time (91). Transformations are insignificant in the lithosphere and atmosphere. The amount of interchange of nitrogen among the lithosphere (not including soil), atmosphere, and living organisms is very small.
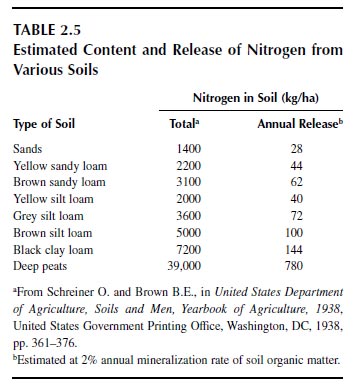
The total amount of nitrogen in the soil to the depth of plowing is considerable relative to the amounts required for crop production, often above 3000 kg/ha but ranging from 1600 kg/ha in sands through 8100 kg/ha in black clay loams to 39,000 kg/ha in deep peats (Table 2.5) (92). Note that the nitrogen in the atmosphere above a hectare of land exceeds 100 million kg at sea level. When land is put for crop production, the nitrogen content of soils declines to a new equilibrium value (90,92). Crop production that relies on the reserves of nitrogen cannot be effective for long, as the reserves become exhausted. Most plants cannot tap into the large reserve of nitrogen in the atmosphere, although biological nitrogen fixation is a means of enhancing the nitrogen content of soils. Biological nitrogen fixation is the principal means of adding nitrogen to the soil from the atmosphere (89). More than 70% of the atmospheric nitrogen added or returned to soils is by biological fixation, and can exceed 100 kg of nitrogen addition per year by nitrogen-fixing legumes.
Most of this nitrogen enters into the organic fraction of the soils. Unless nitrogen-fixing legumes are grown, the addition of nitrogen to soils by biological fixation, averaging about 9.2 kg/ha annually, is too small to support crop production. The remainder is from atmospheric precipitation of ammonium, nitrate, nitrite, and organically bound nitrogen (terrestrial dust). The amount of nitrogen precipitated is normally too small to support crop production but might be of significance in natural landscapes (90). Virtually no interchange of nitrogen occurs between rocks and soils.
 |
Organic Nitrogen in Soil
The concentrations of nitrogen range from 0.02% in subsoils to 2.5% in peats (93). Nitrogen concentrations in soils generally fall sharply with depth, with most of the nitrogen being in the top onemeter layer of soils (89). Surface layers (A-horizon, plow-depth zone) of cultivated soils have between 0.08 and 0.4% nitrogen. Well over 90%, perhaps over 98%, of the nitrogen in the surface layers (A-horizon, plow-depth zone) of soil is in organic matter (93,94). Since most of the nitrogen in soil is organic, determination of total nitrogen has been a common method of estimating organic nitrogen. The Kjeldahl method, a wet digestion procedure (93,95,96), provides a good estimate of organic, soil nitrogen in surface soils, even though some forms of nitrogen (fixed ammonium, nitrates, nitrites, some organic forms) are not determined by this analysis. In depths below the A-horizon or plow zone, although the amounts of total nitrogen are small, inorganic nitrogen, particularly fixed ammonium, is a high proportion of the total, perhaps 40%, and results from Kjeldahl analysis should be treated with some caution as this fraction would not be determined (93). The Dumas method, a dry digestion procedure, is seldom used for determination of nitrogen in soils but generally gives results in close agreement with Kjeldahl determinations, if certain precautions are taken in the analysis (93).Soil organic matter is a complex mixture of compounds in various states of decay or stability (97). Soil organic matter may be classified into humic and nonhumic fractions, with no sharp demarcation between the two fractions. The partially decayed or nonhumic portion is the major source of energy for soil organisms. Depending on the nature of the plant materials, about half of fresh plant residues added to soil decompose in a few weeks or months (98,99). Humus, or humic substances, are the degradation products or residues of microbial action on organic matter and are more stable than the nonhumic substances. Humus is classified into three fractions, humin, humic acids, and fulvic acids, based on their solubilities. Humin is the highest molecular weight material and is virtually insoluble in dilute alkali or in acid. Humic acids are alkali-soluble and acid-insoluble. Fulvic acids are alkalior acid-soluble. The humic and fulvic fractions are the major portions, perhaps 90%, of the humic soil organic matter and are the most chemically reactive substances in humus (100). Humus is slow to mineralize, and unless present in large quantities may contribute little to plant nitrogen nutrition in most soils. About 60 to 75% of the mineralized nitrogen may be obtained by a crop (99). The turnover rate of nitrogen in humus may be about 1 to 3% of the total nitrogen of the soil, varying with type of soil, climate, cultivation, and other factors (93,99). The mineralization rate is likely to be closer to 1% than to 3%. Bremner (96) and Stanford (101) discussed several methods to assess availability of organic nitrogen in soils. Among these procedures were biochemical methods (estimation of microbial growth, mineral nitrogen formed, or carbon dioxide released) and chemical methods (estimation of soil total nitrogen, mineral nitrogen, and organic matter and application of various extraction procedures). The chemical methods are applied more commonly than the biological methods in the estimation of mineralization. Correlation of crop yields to estimations of mineralization generally have not been satisfactory in the assessment of the potential for soils to supply nitrogen for crop growth.
Most studies on the fractionation of total soil organic matter have dealt with the hydrolysis of nitrogenous components with hot acids (3 or 6 M hydrochloric acid for 12 to 24 h) (Table 2.6). The fraction that is not hydrolyzed is called the acid-insoluble nitrogen. The acid-soluble nitrogen is fractionated into ammonium, amino acid, amino sugar, and unidentified components. The origins and composition of each of the named fractions are not clear. The absolute values vary with soil type and with cultivation (94). All of these forms of nitrogen, including the acid-stable form, appear to be biodegradable and, hence, to contribute to plant nutrition (94,102). Organic matter that is held to clays is recalcitrant to biodegradation and increases in relative abundance in heavily cropped soils (94,103,104). This fraction may have little importance in nitrogen nutrition of plants.
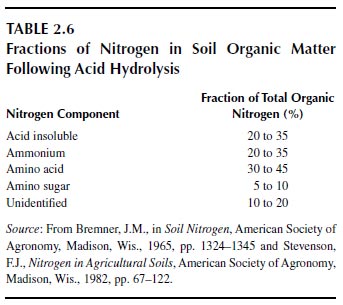 |
Cultivation reduces the total amount of organic matter in soils but has little effect on the relative distribution of the organic fractions in soils, suggesting that the results of acid hydrolysis are of little value as soil tests for available nitrogen or for predicting crop yields (94). Humic substances contain about the same forms of nitrogen that are obtained from the acid hydrolysis of soils but perhaps in different distribution patterns (94). Agricultural systems that depend on soil reserves do not remain productive without the input of fertilizer nitrogen.
Inorganic Nitrogen in Soil
Soil inorganic nitrogen is commonly less than 2% of the total nitrogen of surface soils and undergoes rapid changes in composition and quantity. Inorganic nitrogen varies widely among soils, with climate, and with weather. In humid, temperate zones, soil inorganic nitrogen in surface soil is expected to be low in winter, to increase in spring and summer, and to decrease with fall rains, which move the soluble nitrogen into the depths of the soil (105). Despite being small in magnitude, the inorganic fraction is the source of nitrogen nutrition for plants. Unless supplied by fertilizers, inorganic nitrogen in soil is derived from the soil organic matter, which serves as a reserve of nitrogen for plant nutrition. Plant-available nitrogen is released from organic matter by mineralization and is transformed back into organic matter (microbial cells) by immobilization. Absorption by plants is the chief means of removal of inorganic nitrogen from soils, although nitrate leaching and denitrification, ammonium volatilization and fixation, and nitrogen immobilization lead to losses of inorganic nitrogen from soils or from the soil solution (105).Detectable inorganic nitrogen forms in soil are nitrate, nitrite, exchangeable and fixed ammonium, nitrogen (N2) gas, and nitrous oxide (N2O gas) (106). Nitrate and exchangeable ammonium are important in plant nutrition. The other forms are generally not available for plant nutrition. Fixed ammonium, entrapped in clays, is a principal nitrogenous constituent of subsoils and is probably derived from parent rock materials; however, the fixed ammonium in surface soils may be of recent origin from organic matter (106). Fixed ammonium is resistant to removal from clay lattices and has little importance in plant nutrition. The gaseous constituents diffuse from the atmosphere or arise from denitrification and have no role in plant nutrition, other than in considerations of losses of nitrogen from soils (107).
Exchangeable or dissolved ammonium is available to plants, but ammonium concentrations in soils are low, usually in a magnitude of a few mg/kg or kg/ha. In well-aerated soils, ammonium is oxidized rapidly to nitrate by nitrification, so that nitrate is the major source of plant-available nitrogen in soil (108,109). Nitrite, an intermediate in nitrification, is oxidized more rapidly than ammonium (109). Hence, little ammonium or nitrite accumulates in most soils. Ammonium and nitrite are toxic to most plants (110). Toxicity of ammonium or nitrite might occur if the concentration of either rises above 50 mg N/kg in soil or in other media, especially if either is the principal source of nitrogen for plant nutrition (110,111). Nitrification is sensitive to soil acidity and is likely to be inhibited in soils under pH 5; this acidity may lead to ammonium accumulation (108).




