Properties and Use of Nitrogen Fertilizers
The nitrogen concentrations of the following fertilizers have been rounded to values of commonly marketed grades.Anhydrous Ammonia: 82% N
Anhydrous ammonia is the most-used nitrogen-containing fertilizer for direct application to land in the United States (152). Worldwide, consumption of anhydrous ammonia is ranked fourth or fifth among nitrogen fertilizers (Table 2.7). In agriculture, anhydrous gaseous ammonia is compressed into a liquid and is applied under high pressure with a special implement by injection at least 15cm deep into a moist soil. The ammonia gas reacts with water to form ammonium ions, which can be held to clay or organic matter. If the ammonia is not injected deeply enough or soil is too wet or dry, ammonia can be lost by volatilization. Anhydrous ammonia is usually the cheapest source of nitrogen, but equipment and power requirements of the methods of application are specific and high.Aqua Ammonia: 21% N
Aqua ammonia is ammonia dissolved in water under low pressure. Aqua ammonia must be incorporated into land to avoid losses of nitrogen by ammonia volatilization; however, it needs not be incorporated as deeply as anhydrous ammonia.Urea: 46% N
Urea is the most widely used dry nitrogen fertilizer in the world (Table 2.7). After application to soils, urea is converted into ammonia, which can be held in the soil or converted into nitrate. Ammonia volatilization following fertilization with urea can be substantial, and if urea is applied to the surface of the land, considerable loss of nitrogen can occur (172,173). Hydrolysis of urea by urease produces ammonium carbonate. With surface-applied urea, alkalinity of pH 9 or higher can develop under the urea granule or pellet, and ammonia will volatilize into the air. Volatilization occurs on bare ground, on debris, or on plant leaves. Urea is readily soluble in water, and rainfall or irrigation after its application move it into the soil and lessens volatilization losses. Use of urease inhibitors has been suggested to lessen the volatilization losses of ammonia from surface-applied urea (174). Manufactured urea is identical to urea in animal urine. |
Calcium nitrate urea (calurea, 34% N, 10% Ca) is a double-compound fertilizer of calcium nitrate and urea to supply calcium and nitrogen (152).
Several derivatives of urea are marketed as slow-release fertilizers (175,176). Urea formaldehyde (ureaform, 38% N) is a slow-release fertilizer manufactured from urea and formaldehyde and is used for fertilization of lawns, turf, container-grown plants, and field crops (177–180). Urea formaldehyde is also a glue and is used for the manufacture of plywood and particle board (181,182). Dicyandiamide (cyanoguanidine) (66% N) is a nitrogen fertilizer but is used most commonly as an additive (2% of the total N fertilizer) as a nitrification inhibitor with urea (153,183–185). Sulfur-coated urea (186,187) is a slow-release formulation (30–40% N) used as a fertilizer for field crops, orchards, and turfgrass (175,177,188–191).
Isobutylidene diurea (IBDU) is similar to urea formaldehyde, but contains 32% nitrogen. However, utilization of IBDU is less dependent on microbial activity than urea formaldehyde, as hydrolysis proceeds rapidly following dissolution of IBDU in water (175). Nitrogen is released when soil moisture is adequate. IBDU is used most widely as a lawn fertilizer (176,192). Its field use is to restrict leaching of nitrogen (181).
Methylene ureas are a class of sparingly soluble products, which were developed during the 1960s and 1970s. These products contain predominantly intermediate chain-length polymers. The total nitrogen content of these polymers is 39 to 40%, with between 25 and 60% of the nitrogen present as cold-water-insoluble nitrogen. This fertilizer is used primarily in fertilization of turfgrass, although it has been used with other crops on sandy soils or where leaching of nitrate is an environmental concern (176,191,193).
Ammonium Nitrate: 34% N
Ammonium nitrate is a dry material sold in granular or prilled form. It can be broadcasted or sidedressed to crops and can be left on the surface or incorporated. It does not give an alkaline reaction with soils; hence, it does not volatilize readily. However, incorporation is recommended with calcareous soils. Ammonium nitrate is decreasing in popularity because of storage problems, e.g., with fire and explosion.Calcium ammonium nitrate (ammonium nitrate limestone, about 20% N and 6% Ca) is a mixture of ammonium nitrate and limestone. This fertilizer is not acid-forming and is used to supply nitrogen and calcium to crops (152).
Ammonium Sulfate: 21% N
Ammonium sulfate is marketed as a dry crystalline material. It is recommended for use on alkaline soils where it may be desirable to lower soil pH. Nitrification of ammonium is an acidifying process. Ammonium sulfate can be broadcasted or sidedressed. It can left on surfaces or incorporated, although on calcareous soils watering in or incorporating is recommended to avoid ammonia volatilization (176).Nitrogen Solutions: 28–32% N
These fertilizers are mixtures of ammonium nitrate and urea dissolved in water. In the solutions, half of the nitrogen is supplied as urea, and half is supplied as ammonium nitrate. Because of the difficulties in handling, urea and ammonium nitrate should not be mixed together in dry form. The solution acts once the dry materials are applied to the soil. Ammonia volatilization may be substantial during warm weather, especially with surface application. The solutions should be watered into the soil and should not be applied to foliage.
Ammonium Phosphates: 10–21% N
Ammonium phosphates are important phosphorus-containing fertilizers because of their high concentrations of phosphorus and water solubility. Diammonium phosphate (commonly 18% N, 46% P2O5) is a dry granular or crystalline material. It is a soil-acidifying fertilizer and is useful on calcareous soils. It should be incorporated into the soil. It is a common starter fertilizer and is a common component of greenhouse and household fertilizers. Monoammonium phosphate (commonly 11% N, 48% P2O5) has uses similar to those of diammonium phosphate. Ammonium polyphosphate (10% N, 34% P2O5) is marketed as a solution. Its use is similar to that of monoammonium phosphate and diammonium phosphate. Ammonium phosphates are made by reaction of ammonia with orthophosphoric acid (mono- and diammonium salts) or with superphosphoric (pyrophosphoric) acid (152).Other Inorganic Nitrogen Fertilizers
Many other nitrogen-containing fertilizers include double-salt mixtures such as ammonium nitrate sulfate (30% N), ammonium phosphate nitrate (25% N), urea ammonium phosphate (25–34% N), nitric phosphate, and ammoniated superphosphate (8% N) (152). These materials are used in the manufacture of mixed N-P-K fertilizers or for special needs in soil fertility.Organic Nitrogen Fertilizers: 0.2–15% N
Although naturally occurring, sodium nitrate may not be recognized as an organic fertilizer. Most organic fertilizers are derived from plant and animal sources and are proteinaceous materials. The fertilizer industry started with meat and other food processors, who wanted to dispose of and find a use for wastes and by-products (152,194). Around 1900, about 90% of nitrogen fertilizer was derived from proteinaceous wastes and by-products, but today usage has declined to less than 1%. Organic materials range from less than 1 to about 15% N compared with the chemical sources described above, which range upward to over 80% N. Costs of handling, shipping, and spreading of the bulky, low-analysis organic materials have led to their decline in usage with time. Also, many of the proteinaceous by-products of food processing have higher value as feeds for poultry and livestock than as fertilizers (194,152). Nevertheless, demand for organic fertilizers remains, as organic farmers require these products in the maintenance of soil fertility on their cropland (195).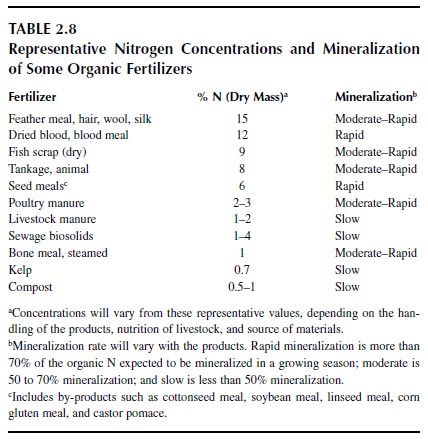 |
The value of organic nitrogen fertilizers depends on their rate of mineralization, which is closely related to their nitrogen concentration (152,195,196). Generally, the more nitrogen in the fertilizer, the faster the rate of mineralization. Some common organic fertilizers are listed in Table 2.8.




