Proteins and Other Nitrogenous Compounds
Unlike animals, plants do not eliminate nitrogen from their bodies but reuse nitrogen from the cycling of proteins and other nitrogenous constituents. Nitrogen losses from plants occur mainly by leaching of foliage by rain or mist and by leaf drop (29). Nitrogen in plants is recycled as ammonium. In the case of hydrolysis (breakdown) of proteins, the amino acids of proteins do not accumulate, but rather nitrogen-rich storage compounds (amides, arginine, and others) accumulate as reserves of nitrogen at the oxidation-reduction level of ammonium. These compounds are formed from the catabolism of proteins. The carbon and hydrogen of proteins are released as carbon dioxide and water. These nitrogen-rich products also accumulate if accumulation of nitrogenous compounds occurs in excess of their conversion into proteins. The amino acids that enter into proteins are not mingled with the storage reserves or translocated products but are made at the same site where protein synthesis occurs.The carbon framework (carbon skeletons) remaining after the donation of nitrogen (ammonium) for amino acid synthesis for incorporation into proteins is metabolized into carbon dioxide and water. Thus, the products of protein catabolism are ammonium, carbon dioxide, and water. Protein turnover (breakdown and resynthesis) may occur in plants in a diurnal cycle, with synthesis occurring in the light and breakdown occurring in the dark, or anabolism and catabolism of proteins may proceed in different compartments of the same cell at the same time (29–31). In a 24-h period, one quarter of the protein in a healthy leaf may be newly synthesized as a result of protein turnover. Most authors indicate a protein turnover of 0.1 to 2% per hour (32,33). With Lemma minor, Trewavas (34,35) measured turnover rates of 7% per day. In an excised leaf, protein synthesis does not proceed after protein hydrolysis, and soluble nitrogenous compounds accumulate. In a nitrogen-deficient plant, the nitrogen will be translocated to a site of need. Also, under normal conditions, leaves will donate some of their nitrogen in leaf proteins to fruits and seeds.
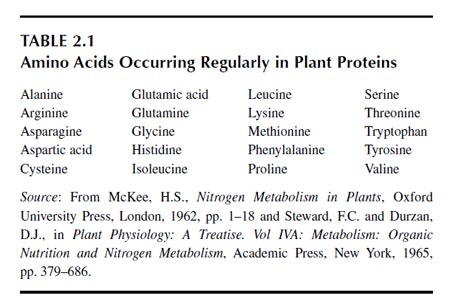
Amino acids are assimilated into proteins or other polypeptides (28). Although plants contain more than 100 amino acids (1,29), only about 20 enter into proteins (Table 2.1). Hydroxyproline may be formed after incorporation of proline into proteins. Cystine is the dimer of cysteine and is formed after incorporation of cysteine into protein. Animal proteins occasionally contain amino acids other than those listed in Table 2.1.
 |
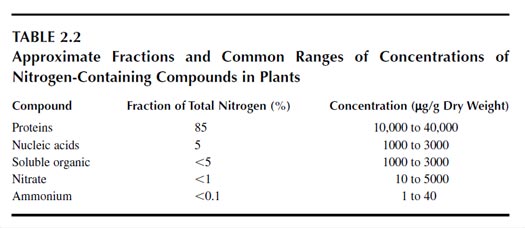 |
The major portion of nitrogen in plants is in proteins, which contain about 85% of the total nitrogen in plants (Table 2.2). Nucleic acids (DNA, RNA) contain about 5% of the total nitrogen, and 5 to 10% of the total nitrogen is in low-molecular-weight, water-soluble, organic compounds of various kinds (36).
Some of the low-molecular-weight, water-soluble, organic compounds are intermediates in the metabolism of nitrogen. Some have specific roles in processes other than intermediary metabolism. Amides and amino acids have roles in transport and storage of nitrogen in addition to their occurrence in proteins. Ureides (allantoin and allantoic acid) are prominent in xylem sap and transport nitrogen fixed in root nodules of legumes (15,29). Amines (ethanolamine) and polyamines (putrescine, spermine, spermidine) have been assigned roles or have putative roles in the lipid fraction of membranes, as protectants, and in processes involved in plant growth and development (15,37–43). Putrescine accumulation in plants may be a physiological response to stresses such as the form of nitrogen supplied and the nutrient status of plants (39,44–46). Simple nitrogen bases, such as choline, are related to alkaloids in plants and to lipids (29). Analogs of purines and pyrimidines have functions in growth regulation (29). Various amino acids other than those in proteins exist in plants. Often, the nonprotein amino acids are related to those occurring in proteins. β-Alanine, homoserine, and &lambda-aminobutyric acid are common examples of these amino acids (1,29). Accumulation of amino acids such as ornithine and citrulline is generally rare in plants, but they may be the major soluble nitrogenous constituents of some species (1). Nonprotein amino acids may be natural products or metabolites, but their functions are generally unclear.




