Function in Plants
Enzyme Activation
The function of potassium in enzyme activation was considered in the preceding section.Protein Synthesis
A probable function of potassium is in polypeptide synthesis in the ribosomes, since that process requires a high K+ concentration (12). Up to now, however, it is not clear which particular enzyme or ribosomal site is activated by K+. There is indirect evidence that protein synthesis requires K+ (13). Salinity from Na+ may affect protein synthesis because of an insufficient K+ concentration in leaves and roots, as shown in Table 4.2 (14). Sodium chloride salinity had no major impact on the uptake of 15N-labelled inorganic N but severely depressed its assimilation and the synthesis of labelled protein. In the treatment with additional K+ in the nutrient solution, particularly in the treatment with 10mM K+, assimilation of inorganic N and protein synthesis were at least as good as in the control treatment (no salinity). In the salinity treatment without additional K+, the K+ concentrations in roots and shoots were greatly depressed. Additional K+ raised the K+ concentrations in roots and shoots to levels that were even higher than the K+ concentration in the control treatment, and at this high cytosolic K+ level, protein synthesis was not depressed.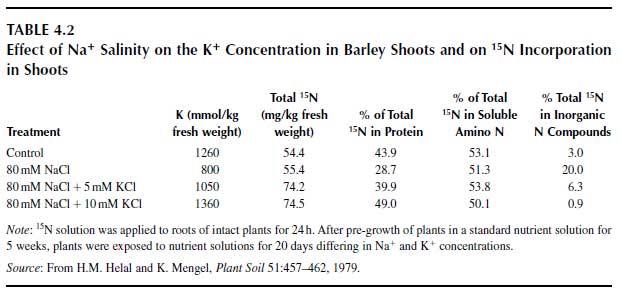 |
Ion Absorption and Transport
Potassium Absorption
Plant membranes are relatively permeable to K+ due to various selective K+ channels across the membrane. Basically, one distinguishes between low-affinity K+ channels and high-affinity channels. For the function of the low-affinity channels, the electrochemical difference between the cytosol and the outer medium (liquid in root or leaf apoplast) is of decisive importance. The K+ is imported into the cell for as long as the electrochemical potential in the cytosol is lower than in the outer solution. With the import of the positive charge (K+) the electrochemical potential increases (decrease of the negative charge of the cytosol) and finally attains that of the outer medium, equilibrium is attained, and there is no further driving force for the uptake of K+ (15). The negative charge of the cytosol is maintained by the activity of the plasmalemma H+ pump permanently excreting H+ from the cytosol into the apoplast and thus maintaining the high negative charge of the cytosol and building up an electropotential difference between the cytosol and the apoplast in the range of 120 to 200mV. If the plasmalemma H+ pumping is affected (e.g., by an insufficient ATP supply), the negative charge of the cytosol drops, and with it the capacity to retain K+, which then streams down the electrochemical gradient through the low-affinity channel, from the cytosol and into the apoplast.Thus in roots, K+ may be lost to the soil, which is, for example, the case under anaerobic conditions. This movement along the electrochemical gradient is also called facilitated diffusion, and the channels mediating facilitated diffusion are known as rectifying channels (16). Inwardly and outwardly directed K+ channels occur, by which uptake and retention of K+ are regulated (17). Their 'gating' (opening and closure) are controlled by the electropotential difference between the cytosol and the apoplast. If this difference is below the electrochemical equilibrium, which means that the negative charge of the cytosol is relatively low, outwardly directed channels are opened and vice versa. The plasmalemma H+-ATPase activity controls the negative charge of the cytosol to a high degree since each H+ pumped out of the cytosol into the apoplast results in an increase of the negative charge of the cytosol. Accordingly, hampering the ATPase (e.g., by low temperature) results in an outwardly directed diffusion of K+ (18). Also, in growing plants, darkness leads to a remarkable efflux of K+ into the outer solution, as shown in Figure 4.2. Within a period of 4 days, the K+ concentration in the nutrient solution in which maize seedlings were grown increased steadily under dark conditions, whereas in light it remained at a low level of <10 �M (19). The outwardly directed channels may be blocked by Ca2+ (20). The blocking may be responsible for the so-called Viets effect (21), which results in an enhanced net uptake of potassium through a decrease in K+ efflux (22).
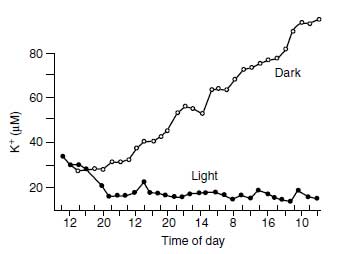 |
| FIGURE 4.2 Potassium concentration changes in the nutrient solution with young intact maize plants exposed to light or dark over 4 days. (Adapted from K. Mengel, in Frontiers in Potassium Nutrition: New Perspectives on the Effects of Potassium on Physiology of Plants. Norcross, GA: Potash and Phosphate Institute, 1999, pp. 1–11.) |
Potassium Transport within Tissues
Opening and closure of K+ channels are of particular relevance for guard cells (23), and the mechanism of this action is controlled by the reception of red light, which induces stomatal opening (24). Diurnal rhythms of K+ uptake were also found by Le Bot and Kirkby (25) and by MacDuff and Dhanoa (26), with highest uptake rates at noon and lowest at midnight. Energy supply is not the controlling mechanism, which still needs elucidation (26). Owing to the low-affinity channels, K+ can be quickly transported within a tissue, and also from one tissue to another. This feature of K+ does not apply for other plant nutrients. The low-affinity channel transport requires a relatively high K+ concentration in the range of >0.1 mM (17). This action is mainly the case in leaf apoplasts, where the xylem sap has K+ concentrations >1 mM (27). At the root surface, the K+ concentrations may be lower than 0.1mM, and here high-affinity K+ channels are required, as well as low-affinity channels, for K+ uptake.The principle of high-affinity transport is a symport or a cotransport, where K+ is transported together with another cationic species such as H+ or even Na+. The K+-H+ or K+-Na+ complex behaves like a bivalent cation and has therefore a much stronger driving force along the electrochemical gradient. Hence, K+ present near the root surface in micromolar concentrations is taken up.
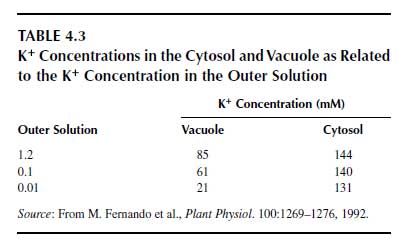
Because of these selective K+ transport systems, K+ is taken up from the soil solution at high rates and is quickly distributed in plant tissues and cell organelles (28). Potassium ion distribution in the cell follows a particular strategy, with a tendency to maintain a high K+ concentration in the cytosol, the so-called cytoplasmic potassium homeostasis, and the vacuole functions as a storage organelle for K+ (29). Besides the H+-ATPase, a pyrophosphatase (V-PPase) is also located in the tonoplast, for which the substrate is pyrophosphate. The enzyme not only pumps H+ but also K+ into the vacuole, and thus functions in the cytoplasmic homeostasis (Figure 4.3). This mechanism is an uphill transport because the vacuole liquid is less negatively charged than the cytosol. In Table 4.3, the typical pattern of K+ concentration in relation to K+ supply is shown (30). The cytosolic K+ concentration remains at a high level almost independently of the K+ concentration in the nutrient solution, whereas the vacuolar K+ concentration reflects that of the nutrient solution.
Osmotic Function
The high cytosolic K+ concentration required for polypeptide synthesis is particularly important in growing tissues; the K+ in the vacuole not only represents K+ storage but also functions as an indispensable osmoticum. In most cells, the volume of the vacuole is relatively large, and its turgor is essential for the tissue turgor. The osmotic function is not a specific one as there are numerous organic and inorganic osmotica in plants. There is a question, however, as to whether these can be provided quickly to fast-growing tissues, and in most cases it is the K+ that is delivered at sufficient rates. In natrophilic species, Na+ may substitute for K+ in this osmotic function. The high vacuolar turgor in expanding cells produces the pressure potential required for growth. This pressure may be insufficient (p<0.6MPa) in plants suffering from K+ deficiency (31). In Figure 4.4, pressure potentials and the related cell size in leaves of common bean (Phaseolus vulgaris L.) are shown. Pressure potentials (turgor) were significantly higher in the treatment with sufficient K+ compared with insufficient K+ supply. This higher turgor (Ψp) promoted cell expansion, as shown in the lower part of Figure 4.4. From numerous observations, it is well known that plants insufficiently supplied with K+ soon lose their turgor when exposed to water stress. In recent experiments it was found that K+ increased the turgor and promoted growth in cambial tissue (32). The number of expanding cells derived from cambium was reduced with insufficient K+ nutrition.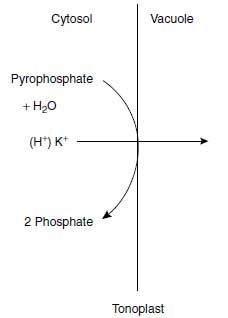 |
| FIGURE 4.3 Pyrophosphatase located in the tonoplast and pumping H+ or K+ from the cytosol into the vacuole. |
 |
Photosynthesis and Respiration
Potassium ion transport across chloroplast and mitochondrial membranes is related closely to the energy status of plants. In earlier work, it was shown that K+ had a favorable influence on photoreduction and photophosphorylation (33). More recently, it was found that an ATPase located in the inner membrane of chloroplasts pumps H+ out of the stroma and thus induces a K+ influx into the stroma via selective channels (34). The K+ is essential for H+ pumping by the envelope-located ATPase (35). Were it not for a system to pump H+ from the illuminated chloroplast, the increase in stromal pH induced by the electron flow in the photosynthetic electron-transport chain would quickly dissipate (34). This high pH is a prerequisite for an efficient transfer of light energy into chemical energy, as was shown by a faster rate of O2 production by photolysis in plants treated with higher K+ concentration (36). The favorable effect of K+ on CO2 assimilation is well documented (37,38). An increase in leaf K+ concentration was paralleled by an increase in CO2 assimilation and by a decrease in mitochondrial respiration (38). Obviously, photosynthetic ATP supply substituted for mitochondrial ATP in the leaves with the high K+ concentration. Thus, K+ had a beneficial impact on the energy status of the plant.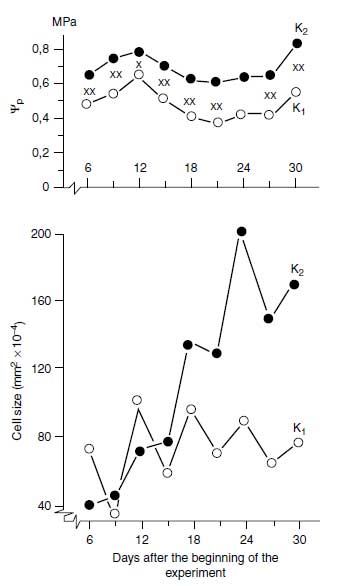 |
| FIGURE 4.4 Pressure potential (φp) and cell size in leaves of common bean (Phaseolus vulgaris L.) insufficiently (K1) and sufficiently (K2) supplied with K+. (Adapted from K. Mengel and W.W. Arneke, Physiol. Plant 54:402–408, 1982.) |
Long-Distance Transport
Long-distance transport of K+ occurs in the xylem and phloem vessels. Loading of the xylem occurs mainly in the root central cylinder, where protoxylem and xylem vessels are located adjacent to xylem parenchyma cells. The K+ accumulates in the parenchyma cells (Figure 4.5) and is transported from there across the plasmalemma and the primary cell wall and through pits of the secondary cell wall into the xylem vessels (39). There is evidence that the outward-rectifying channels allow a K+ flux (facilitated diffusion) from the parenchyma cells into the xylem vessel (40,41). The release of K+ into the xylem sap decreases its water potential and thus favors the uptake of water (42). The direction of xylem sap transport goes along the transpiration stream and hence from root to leaves. The direction of the phloem sap transport depends on the physiological conditions and goes toward the strongest sinks. These may be young growing leaves, storage cells of roots, or fleshy fruits like tomato.Phloem sap is rich in K+, with a concentration range of 60 to 100 mM (43). Potassium ions are important for phloem loading and thus phloem transport. It was shown that K+ particularly promotes the uptake of sucrose and glutamine into the sieve cells at high apoplastic pH (44). These metabolites presumably are taken up into the sieve vessels via a K+ cotransport (Figure 4.5). This process is important, since in cases in which insufficient H+ are provided by the plasmalemma H+ pump, and thus the apoplastic pH is too high for a H+ cotransport of metabolites, K+ can substitute for H+ and the most important metabolites required for growth and storage, sucrose and amino compounds, can be transported along the phloem. Hence the apoplastic K+ concentration contributes much to phloem loading (Figure 4.5). This occurrence is in line with the observation that the phloem flow rate in castor bean (Ricinus communis L.) was higher in plants well supplied with K+ than in plants with a low K+ status (43). The favorable effect of K+ on the transport of assimilates to growing plant organs has been shown by various authors (45).
Potassium ions cycle via xylem from roots to upper plant parts and via phloem from leaves to roots. The direction depends on the physiological demand. During the vegetative stage, the primary meristem is the strongest sink. Here, K+ is needed for stimulating the plasmalemma ATPase that produces the necessary conditions for the uptake of metabolites, such as sucrose and amino acids. High K+ concentrations are required in the cytosol for protein synthesis and in the vacuole for cell expansion (Figure 4.4). During the generative or reproductive phase, the K+ demand depends on whether or not fruits rich in water are produced, such as apples or vine berries. These fruits need K+ mainly for osmotic balance. Organs with a low water content, such as cereal grains, seeds, nuts, and cotton bolls, do not require K+ to a great extent. Provided that cereals are well supplied with K+ during the vegetative stage, K+ supply during the generative stage has no major impact on grain formation (46).
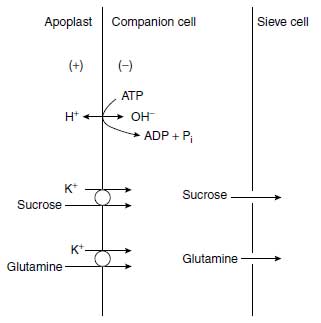 |
| FIGURE 4.5 Cotransport of K+/sucrose and K+/glutamine from the apoplast into the companion cell, and from there into the sieve cell, driven by the plasmalemma ATPase. |
However, for optimum grain filling, a high K+ concentration in the leaves is required for the translocation of assimilates to the grains and for protein synthesis in these grains (47).
The generative phase of cereal growth requires hardly any K+, but still appreciable amounts of N. In such cases, nitrate uptake of the plants is high and K+ uptake low. The K+ is recycled via the phloem from the leaves to the roots, where K+ may enter the xylem again and balance the negative charge of the NO3- (48). Both the ionic species, K+ and nitrate, are efficient osmotica and are thus of importance for the uptake of water into the xylem (49). In the phloem sap, K+ balances the negative charge of organic and inorganic anions.
In storage roots and tubers, K+ is required not only for osmotic reasons, but it may also have a more specific function. From work with sugar beet (Beta vulgaris L.) roots, a K+-sucrose cotransport across the tonoplast into the vacuole, driven by an H+/K+ antiport cycling the K+ back into the cytosol, was postulated (50).




