Potassium Bearing Minerals
The average potassium concentration of the earth’s crust is 23 g/kg. Total potassium concentrations in the upper soil layer are shown for world soils and several representative soil groups in Table 4.7 (78). The most important potassium-bearing minerals in soils are alkali feldspars (30 to 20 g K/kg), muscovite (K mica, 60 to 90 g K/kg), biotite (Mg mica, 36 to 80 g K/kg), and illite (32 to 56 g K/kg). These are the main natural potassium sources from which K+ is released by weathering and which feed plants. The basic structural element of feldspars is a tetrahedron forming a Si—Al–O framework in which the K+ is located in the interstices. It is tightly held by covalent bonds (79). The weathering of the mineral begins at the surface and is associated with the release of K+. This process is promoted by very low K+ concentrations in the soil solution in contact with the mineral surface, and these low concentrations are produced by K+ uptake by plants and microorganisms and by K+leaching. The micas are phyllosilicates (80) and consist of two Si-Al-O tetrahedral sheets between which an M-O-OH octahedral sheet is located. M stands for Al3+, Fe2+, Fe3+, or Mg2+ (81). Because of this 2:1 layer structure, they are also called 2:1 minerals. These three sheets form a unit layer, and numerous unit layers piled upon each other form a mineral. These unit layers of mica and illite are bound together by K+ (Figure 4.8). K+ is located in hexagonal spaces formed by O atoms, of which the outer electron shell attracts the positively charged K+. During this attraction process, the K+ is stripped of its hydration water. This dehydration is a selective process due to the low hydration energy of K+.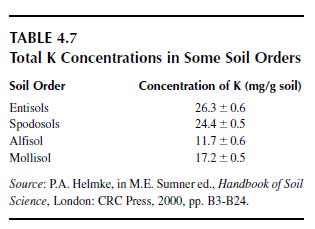 |
This action is in contrast to Na+, which has a higher hydration energy than K+; the hydrated water molecules are bound more strongly and hence are not stripped off, and the hydrated Na+ does not fit into the interlayer. The same holds for divalent cations and cationic aluminum species. This selective K+ bond is the main reason why K+ in most soils is not leached easily, in contrast to Na+. Ammonium has a similar low hydration energy as K+ and can, for this reason, compete with K+ for interlayer binding sites (82,83). This interlayer K+ is of utmost importance for the release and for the storage of K+. Equilibrium conditions exist between the K+ concentration in the adjacent soil solution and the interlayer K+. The equilibrium level differs much between biotite and muscovite, the former having an equilibrium at about 1 mM and the latter at about 0.1mM K+ in the soil solution (84). For this reason, the K+ of the biotite is much more easily released than the K+ from muscovite, and hence the weathering rate associated with the K+ release of biotite is much higher than that of muscovite. The K+ release is induced primarily by a decrease of the K+ concentration in the adjacent solution caused by K+ uptake of plant roots, or by K+ leaching, or by both processes. The release of K+ begins at the edge positions and proceeds into the inner part of the interlayer. This release is associated with an opening of the interlayer because the bridging K+ is lacking.
The free negative charges of the interlayer are then occupied by hydrated cationic species (Ca2+,Mg2+, Na+, cationic Al species). From this process, it follows that the interlayer K+ is exchangeable. The older literature distinguishes between p (planar), e (edge), and i (inner) positions of adsorbed (exchangeable) K+ according to the sites where K+ is adsorbed, at the outer surface of the mineral, at the edge of the interlayer, or in the interlayer. It is more precise, however, to distinguish between hydrated and nonhydrated adsorbed K+ (79), the latter being much more strongly bound than the former. With the exception of the cationic aluminum species, hydrated cationic species may be replaced quickly by K+ originating from the decomposition of organic matter or inorganic and organic (slurry, farm yard manure) K fertilizer. The dehydrated K+ is adsorbed and contracts the interlayers and is thus ‘fixed.’ The process is called K+ fixation. Fixation depends much on soil moisture and is restricted by dry (and promoted by moist) soils.
It is generally believed that H+ released by roots also contributes much to the release of K+ from K-bearing minerals. This process, however, is hardly feasible since in mineral soils the concentration of free protons is extremely low and is not reflected by the pH because of the very efficient H+ buffer systems in mineral soils (85). It is the decrease of the K+ concentration in the adjacent solution that mainly drives the K+ release (86,87). Only high H+ concentrations (pH<3) induce a remarkable release of K+, associated with the decomposition of the mineral (88). A complete removal of the interlayer K+ by hydrated cations, including cationic aluminum species, leads to the formation of a new secondary mineral as shown in Figure 4.8 for the formation of vermiculite from mica (15).
In acid mineral soils characterized by a relatively high concentration of cationic aluminum species, the aluminum ions may irreversibly occupy the interlayer sites of 2:1 minerals, thus forming a new secondary mineral called chlorite. By this process, the soil loses its specific binding sites for K+ and hence the capacity of storing K+ in a bioavailable form.
 |
| FIGURE 4.8 Scheme of a K+-contracted interlayer of mica or illite and of vermiculite interlayer expanded by Mg2+. (Adapted from K. Mengel and E.A. Kirkby, Principles of Plant Nutrition. 5th ed. Dordrecht: Kluwer Academic Publishers, 2001.) |
Under humid conditions in geological times, most of the primary minerals of the clay fraction were converted into secondary minerals because of K+ leaching. The process is particularly relevant for small minerals because of their large specific surface. For this reason, in such soils the clay fraction contains mainly smectites and vermiculite, which are expanded 2:1 clay minerals. In soils derived from loess (Luvisol), which are relatively young soils, the most important secondary mineral in the clay fraction is the illite, which is presumably derived from muscovite. Its crystalline structure is not complete, it contains water, and its K+ concentration is lower than that of mica (89). Mica and alkali feldspars present in the silt and sand fraction may considerably contribute to the K+ supply of plants (90,91). Although the specific surface of these primary minerals in the coarser fractions is low, the percentage proportion of the silt and sand fraction in most soils is high and, hence, also the quantity of potassium-bearing minerals.
Cropping soils without replacing the K+ removed from the soil in neutral and alkaline soils leads to the formation of smectites and in acid soils to the decomposition of 2:1 potassium-bearing minerals (92). Smectites have a high distance between the unit layers, meaning that there is a broad interlayer zone occupied mainly by bivalent hydrated cationic species and by adsorbed water molecules. For this reason, K+ is not adsorbed selectively in the interlayers of smectites. The decomposition of K+-selective 2:1 minerals results also from K+ leaching. In addition, under humid conditions, soils become acidic, which promotes the formation of chlorite from K+-selective 2:1 minerals. Thus, soils developed under humid conditions have a poor K+-selective binding capacity and are low in potassium, for example, highly weathered tropical soils (Oxisols).
Organic soil matter has no specific binding sites for K+, and therefore its K+ is prone to leaching. Soils are generally lower in potassium, and their proportion of organic matter is higher. Soils with a high content of potassium are young soils, such as many volcanic soils, but also include soils derived from loess under semiarid conditions.




