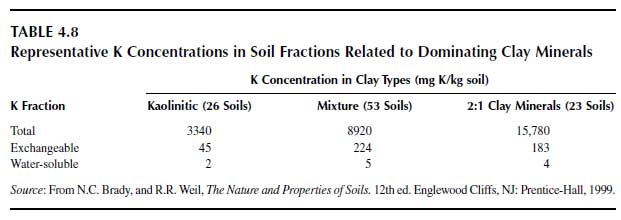
Potassium Fractions in Soils
Fractions of potassium in soil are (a) total potassium, (b) nonexchangeable (but plant-available) potassium, (c) exchangeable potassium, and (d) water-soluble potassium. The total potassium comprises the mineral potassium and potassium in the soil solution and in organic matter. Soil solution potassium plus organic matter potassium represent only a small portion of the total in mineral soils. The total potassium depends much on the proportion of clay minerals and on the type of clay minerals. Kaolinitic clay minerals, having virtually no specific binding sites for K+, have low potassium concentrations in contrast to soils rich in 2:1 clay minerals. Mean total K+ concentrations, exchangeable K+ concentrations, and water-soluble K+ are shown Table 4.8 (93).Soils with mainly kaolinitic clay minerals have the lowest, and those with smectitic minerals, which include also the 2:1 clay minerals with interlayer K+, have the highest potassium concentration. The K+ concentration of the group of mixed clay minerals, kaolinitic and 2:1 clay minerals, is intermediate. Water-soluble K+ depends on the clay concentration in soils and on the type of clay minerals. As can be seen from Figure 4.9, the index of soluble K+ decreases linearly with an increase in the clay concentration in soils and the level of soluble K+ in the kaolinitic soil group is much higher than that of the mixed soil group and of the smectitic soil group (94).
The determination of total soil potassium requires a dissolution of potassium-bearing soil minerals. The digestion is carried out in platinum crucibles with a mixture of hydrofluoric acid, sulfuric acid, perchloric acid, hydrochloric acid, and nitric acid (95). Of particular importance in the available soil potassium is the exchangeable K+, which is obtained by extracting the soil sample with a 1M NH4Cl or a 1M NH4+ acetate solution (96). With this extraction, the adsorbed hydrated K+ and some of the nonhydrated K+ (K+ at edge positions) is obtained. In arable soils, the exchangeable K+ ranges between 40 to 400 mg K/kg. Soil extraction with CaCl2 solutions (125 mM) extracts somewhat lower quantities of K+ as the Ca2+ cannot exchange the nonhydrated K+, in contrast to NH4+of the NH4+-containing extraction solutions.
For the determination of the nonexchangeable K+, not obtained by the exchange with NH4+and consisting of mainly interlayer K+ and structural K+ of the potassium feldspars, diluted acids such as 10 mM HCl (97) or 10mM HNO3 are used (98). These extractions have the disadvantage in that they extract a K+ quantity and do not assess a release rate, the latter being of higher importance for the availability of K+ to plants.
The release of K+ from the interlayers is a first-order reaction (83) and is described by the following equations (99):
. Elovich function: y = a + b ln t
. Exponential function: ln y = ln a + b ln t
. Parabolic diffusion function: y = b t1/2
where y is the quantity of extracted K+, a the intercept on the Y-axis, and b the slope of the curve.
 |
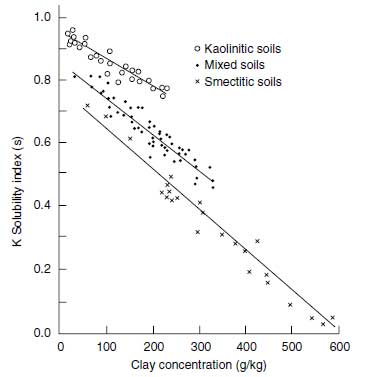 |
| FIGURE 4.9 Potassium solubility of various soils related to their type of clay minerals (Adapted from A.N. Sharpley, Soil Sci. 149:44-51, 1990.) |
In this investigation, soils were extracted repeatedly by Ca2+-saturated ion exchangers for long periods (maximum time 7000 h). Analogous results are obtained with electro-ultra-filtration (EUF), in which K+ is extracted from a soil suspension in an electrical field (100). There are two successive extractions; the first with 200 V and at 20°C (first fraction) and a following extraction (second fraction) with 400 V at 80°C. The first fraction contains the nonhydrated adsorbed K+ plus the K+ in the soil solution, whereas the second fraction contains the interlayer K+. The extraction curves are shown for four different soils in Figure 4.10, from which it is clear that the K+ release of the second fraction is a first-order reaction (101). The curves fit the first-order equation, the Elovich function, the parabolic diffusion function, and the power function, with the Elovich function having the best fit with R2>0.99.




