Secondary Sulfur Compounds
There are more than 100,000 known secondary plant compounds, and for only a limited number of them are the biochemical pathways, functions, and nutritional and medicinal significance known (84). Detailed overviews of the biochemical pathways involved in the synthesis of the sulfur-containing secondary metabolites, glucosinolates and alliins, are provided by Halkier (84) and Lancaster and Boland (85). Bioactive secondary plant compounds comprise various substances such as carotenoids, phytosterols, glucosinolates, flavonoids, phenolic acids, protease inhibitors, monoterpenes, phyto-estrogens, sulfides, chlorophylls, and roughages (87). Often, secondary metabolites are accumulated in plant tissues and concentrations of 1 to 3% dry weight have been determined (88). Secondary compounds in plants usually have a pharmacological effect on humans (87). Therefore, secondary metabolites contribute significantly to food quality, either as nutritives or antinutritives. Plants synthesize a great array of secondary metabolites as they are physically immobile (88), and the presence of secondary compounds may give either repellent or attractant properties.The bioactive components in medicinal plants comprise the whole range of secondary metabolites and crop-specific cultivation strategies, which include fertilization, harvesting, and processing techniques, and which are required for producing a consistently high level of bioactive constituents. Ensuring a consistently high quality of the raw materials can be a problem, particularly if the active agent is unstable and decomposes after harvesting of the plant material, as is true for many secondary metabolites such as the sulfur-containing alliins and glucosinolates (89).
Glucosinolates are characteristic compounds of at least 15 dicotyledonous families. Of these, the Brassicaceae are the most important agricultural crops. Glucosinolates act as attractants, repellents, insecticides, fungicides, and antimicrobial protectors. The principal structure of a glucosinolate is given in Figure 7.4.
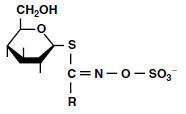 |
| FIGURE 7.4 Basic structure of glucosinolates. (From Schnug, E., in Sulfur Nutrition and Sulfur Assimilation in Higher Plants, SPB Academic Publishing, The Hague, 1990, pp. 97-106.) |
There are about 80 different glucosinolates, which consist of glucose, a sulfur-containing group with an aglucon rest, and a sulfate group (87). Alkenyl glucosinolates such as progoitrin and gluconapin have an aliphatic aglucon rest, whereas indole glucosinolates such as glucobrassicin and 4-hydroxyglucobrassicin in rape (Brassica napus L.) have an aromatic aglucon rest (Figure 7.4). Additional information about the characteristics of glucosinolate side-chains is given by Underhill (91), Larsen (92), and Bjerg et al. (93).
Glucosinolates are generally hydrolyzed by the enzyme myrosinase, which is present in all glucosinolate- containing plant parts. Bones and Rossiter (94) provided basic information about the biochemistry of the myrosinase-glucosinolate system. A proposed pathway for the recyclization of sulfur (and N) under conditions of severe sulfur deficiency is described by Schnug and Haneklaus (53).
The degradation of glucosinolates results in the so-called mustard oils, which are responsible for smell, taste, and biological effect. Glucosinolates are vacuolar defense compounds (95) of qualitative value (96) and are effective against generalist insects at low tissue concentrations (97). Isothiocyanates, the breakdown products after enzymatic cleavage of glucosinolates, may retard multiplication of spores but do not hamper growth of fungal mycelium (98), and fungi may overcome the glucosinolate-myrosinase system efficiently (99,100).
The influence of the sulfur nutritional status on the content of glucosinolates and other sulfurcontaining secondary metabolites, which are related to nutritional and pharmaceutical quality, is shown in Table 7.4.
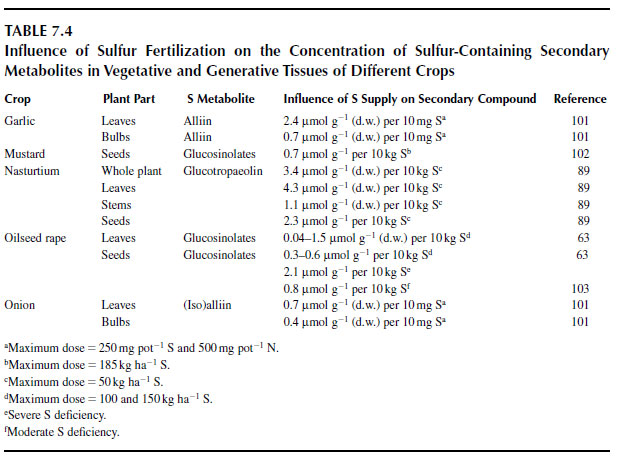 |
Generally, nitrogen fertilization reduces the glucosinolate content (104). However, under field conditions the effect of nitrogen fertilization on glucosinolate content varies substantially between seasons (105). Schnug (103) noted a distinct interaction between nitrogen and sulfur fertilization when nitrogen was supplied insufficiently, whereby the alkenyl, but not the indole, glucosinolate content in seeds of rape increased at higher nitrogen and sulfur rates. Kim et al. (106) also showed that nitrogen fertilization increased the alkenyl-glucosinolates, gluconapin, and glucobrassicanapin in particular, in rape.
More than 80% of the total sulfur in Allium species is present in secondary compounds. Allium species contain four S-alk(en)yl-L-cysteine sulfoxides, namely S-1-propenyl-, S-2-propenyl-, S-methyl- and S-propyl-L-cysteine sulfoxides (107). Iso-alliin is the main form in onions, whereas alliin is the predominant form in garlic (108) (Figure 7.5). Alliins supposedly contribute to the defense of plants against pests and diseases. In vitro and in vivo experiments revealed a bactericidal effect against various plant pathogens (109).
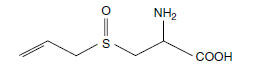 |
| FIGURE 7.5 Chemical structure of alliin. (From Watzl, B., Bioaktive Substanzen in Lebensmitteln, Hippokrates Verlag, Stuttgart, Germany, 1999.) |
The characteristic flavor of Allium species is caused after the enzyme alliinase hydrolyzes cysteine sulfoxides to form pyruvate, ammonia, and sulfur-containing volatiles. In the intact cell, alliin and related cysteine sulfoxides are located in the cytoplasm, whereas the C-S lyase enzyme alliinase is localized in the vacuole (110). Disruption of the cell releases the enzyme, which causes subsequent α,β-elimination of the sulfoxides, ultimately giving rise to volatile and odorous LMW organosulfur compounds (111). The cysteine sulfoxide content of Allium species is an important quality parameter with regard to sensory features, since it determines the taste and sharpness.
Alliin acts as an antioxidant by activating glutathione enzymes and is regarded as having an anticarcinogenic and antimicrobial effect (86). On average, 21% of sulfur, but only 0.9% of nitrogen, are present as (iso)alliin in onion bulbs at the start of bulb growth (101). The ratio between protein-S and sulfur in secondary metabolites of the Allium species is, at between 1:4 and 1:6, much wider than in members of the Brassica family (between 1:0.3 and 1:2). The reason for this difference is supposedly the fact that glucosinolates may be reutilized under conditions of sulfur deficiency whereas alliins are inert end products. Interactions between nitrogen and sulfur supply exist in such a way that nitrogen and sulfur fertilization has been shown to decrease total sulfur and nitrogen concentration, respectively, in onion (101).




