Cutin and Suberin
Functional and Ultrastructural Characteristics
Cutin is the main structural component of the multilayered cuticle that covers all
epidermal cells of the aerial portions of plants as a continuous extracellular layer
of hydrophobic material. Cutin forms, together with the intracuticular waxes in
which it is embedded, the so-called cuticle proper that is overlaid by epicuticular
waxes (Jeffree, 1996). Waxes are complex mixtures of long-chain fatty acids and
their derivatives, but may also contain other embedded compounds, such as
triterpenoids and flavonoids. The cuticle proper is linked via a so-called ‘‘cuticular
layer,’’ also containing polysaccharides, to the cell wall (Jeffree, 1996).
In addition to coating the external epidermal surface, the cuticular membrane
extends into the substomatal chamber (Esau, 1977). The cuticle plays an important role in protecting plants from physical, chemical, and biological aggressions, for
example, ultraviolet (UV) irradiation, mechanical damage, as well as pathogen
and insect attack (Kerstiens, 1996). The cuticle also covers the protoderm of the
embryo, playing an important role during development in the prevention of organ
fusions (Tanaka
et al., 2001).
Suberin is constitutively present in the secondary growth periderm of aerial
tissues and in several underground tissues, for example, epidermis, hypodermis,
peridermis, and the Casparian strips of the root endodermis. It may also be
deposited in bundle sheets, the chalazae, and abscission zone during seed development,
and in secretory organs as well as fibers. Suberin is also produced at
wound sites to replace the missing cuticle (Kolattukudy, 1981). Similar to cutin,
the function of suberin is to seal off the respective tissue to inhibit water loss or
contribute to resistance to pathogen attack.
 |
| FIGURE 8.1 Ultrastructure of the cuticle of the
epidermis of Arabidopsis stems. Cuticle of
amorphous appearance (small arrowheads)
overlaying the cell wall polysaccharides.
Bar = 200 nm. |
|
Despite functional, structural, and chemical similarities of suberin and cutin,
both polymers are characterized by differences in their composition and location
within the plant. While cutin is deposited only on the outside of the epidermal cell
wall in the cuticle, suberin is deposited as part of the primary cell wall close to the
plasma membrane. The ultrastructure of cutin and suberin deposition may also be
different. The ultrastructure of cutin may be of amorphous, recticulate, or lamellate
appearance depending on the plant tissue (Fig. 8.1). This feature is used for
the classification of cutin types (Holloway, 1982). In contrast, the ultrastructure of
suberin is very characteristic, having an alternation of lamellae of electron-opaque
and electron-translucent materials in transmission electron microscopy (TEM)
(Fig. 8.2) (Bernards, 2002; Nawrath, 2002).
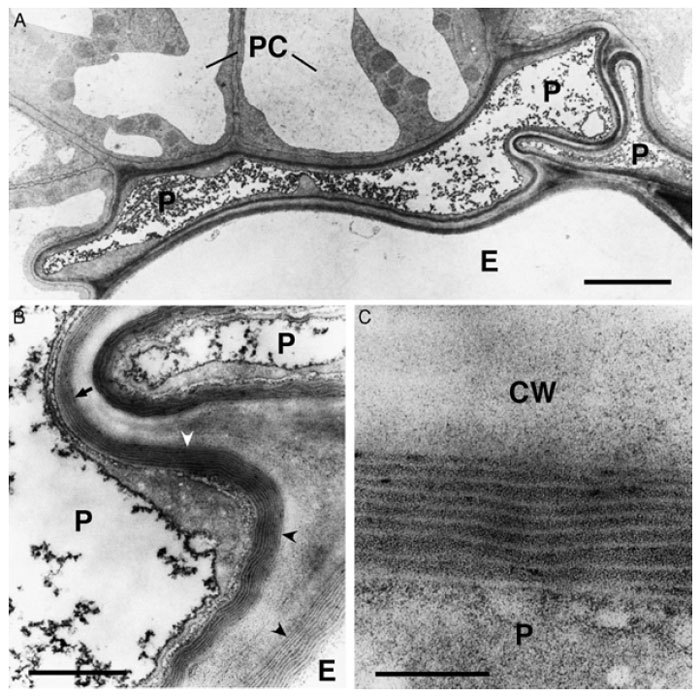 |
| FIGURE 8.2 Ultrastructure of suberized roots tissues of Arabidopsis plants at the beginning of
the secondary thickening of the root. (A) Overview of suberized endodermal and peridermal cells
in the root. The suberin deposition is visible as an electron-opaque layer inside of the primary cell
wall. The fully suberized peridermal cell layer typically collapses during the dehydration and
embedding procedures necessary for TEMbecause of the lowpermeability of the suberized cellwalls.
Bar = 2.5 µm. (B) Enlargement of (A). Fine structure of suberin. The structure of the lamellae with an
alternation of electron-opaque and electron-translucent layers of suberin is clearly visible when the
specimen is cut perpendicularly to the suberin layers (concave arrowheads). However, the lamellate
structure of suberin is barely visible when the specimen in not cut perpendicularly to the suberin
layers (arrow).
Bar = 500 nm. (C) Enlargement of
(B). The thickness of the electron-opaque and
electron-translucent layers of the suberin is very regular and
characteristic for the tissue sample.
Bar = 100 nm. P, peridermal cell; E, endodermal cell; PC, pericycle cell; CW, cell wall. |






