Polyhydroxybutyrate
PHB is the most widespread and thoroughly characterized PHA found in bacteria.
A large part of our knowledge on PHB biosynthesis has been obtained from
R.
eutropha (Steinbü chel and Hein, 2001). In this bacterium, PHB is synthesized
from acetyl-CoA by the sequential action of three enzymes (Fig. 8.4). The first
enzyme of the pathway, 3-ketothiolase, encoded by the
phbA gene, catalyzes
the reversible condensation of two acetyl-CoA moieties to form acetoacetyl-CoA.
Acetoacetyl-CoA reductase, encoded by the
phbB gene, subsequently reduces
acetoacetyl-CoA to R-(—)-3-hydroxybutyryl-CoA, which is then polymerized to
PHB by the action of a PHA synthase encoded by the
phaC gene. The PHA synthase
of R.
eutropha has been shown to accept the R-isomer of 3-hydroxybutyryl-CoA but
not the S-isomer.PHAis typically produced as a polymer of 10
3 to 10
4monomers that
accumulates as inclusions of 0.2 to 0.5 mmin diameter. In R.
eutropha, PHB inclusions can typically accumulate to 80–85% of the dry weight (dwt) when bacteria are
grown in media containing excess carbon, such as glucose, but limited in one
essential nutrient, such as nitrogen or phosphate (Steinbüchel and Schlegel, 1991).
Under these conditions, PHB synthesis acts as a carbon reserve and an electron sink.
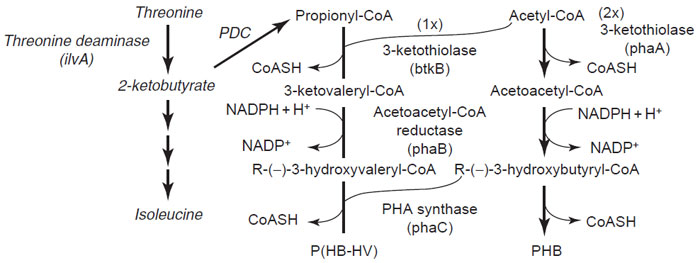 |
| FIGURE 8.4 Pathways of PHB and P(HB-HV) synthesis. The pathways common to bacteria and
transgenic plants are shown in plain letters while the pathway specific to transgenic plants is
shown in italics. PDC refers to the plant endogenous pyruvate dehydrogenase complex. |
PHB is a highly crystalline polymer and a stiff and relatively brittle thermoplastic
(de Koning, 1995). Its melting point (T
m = 175 °C) is only slightly lower
than the temperature at which it starts degrading to crotonic acid, making processing
difficult. These properties seriously limit its use in a wide range of commodity
products. PHB has good UV light resistance but relatively poor resistance to
acids and bases. The polymer is water and air impermeable as well as relatively
resistant to hydrolytic degradation, making it superior to starch-derived plastics,
which are moisture sensitive.
Synthesis of PHB in the Cytoplasm
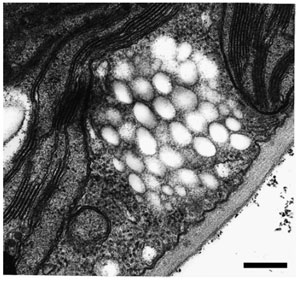 |
| FIGURE 8.5 Accumulation of PHA inclusions in
the cytoplasm of transgenic A. thaliana cells
expressing the PHB biosynthetic pathway.
Bar = 1 µm. |
Despite its relatively poor physical properties as a thermoplastic, PHB was initially
targeted for production in plants because the first bacterial PHA biosynthetic
genes that were cloned were for PHB synthesis in the bacterium
R. eutropha (Schubert
et al., 1988; Slater
et al., 1988). The cytoplasm was targeted as the first
site for PHB synthesis because, in addition to containing acetyl-CoA, the building
block for PHB, it also had the advantage that the bacterial enzymes could be
directly expressed in this compartment without any modification of the proteins.
Furthermore, an endogenous plant 3-ketothiolase is present in the cytoplasm as
part of mevalonate pathway. Thus, creation of the PHB biosynthetic pathway in
the cytoplasm was theoretically simpler, requiring only the expression of two
additional enzymes, the reductase and synthase. The
R. eutropha phaB and
phbC
genes, encoding, respectively, the acetoacetyl-CoA reductase and PHA synthase,
were coexpressed in
A. thaliana under the control of the cauliflower mosaic virus
(CaMV) 35S promoter, allowing a relatively high expression of the enzymes in a
broad range of tissues (Poirier
et al., 1992a). The highest amount of PHB measured
in the shoots of these plants was ~0.1% dwt (Poirier
et al., 1992a). Detailed analysis
of the PHB purified from
A. thaliana confirmed that the polymer was isotactic poly ([R]-(—)-3-hydroxybutyrate) and that the thermal properties of plant PHB were
similar to those of bacterial PHB (Poirier
et al., 1995b). Furthermore, PHB
accumulated in the form of granules that had a size and appearance very similar
to bacterial PHB granules (Fig. 8.5) (Poirier
et al., 1992a).
Plants expressing high level of acetoacetyl-CoA reductase in the cytoplasm
have shown a strong reduction in growth, with the most affected plants being
approximately five times smaller by fresh weight compared to wild-type plants
(Poirier
et al., 1992b). There was an overall good correlation between the extent
of the growth reduction and the level of reductase enzyme activity. While no
abnormal phenotype was observed in plants expressing only the PHB synthase
(and not producing PHB), combination of the acetoacetyl-CoA reductase with
the PHB synthase led to a further reduction in growth compared to plants
expressing only the reductase (Poirier
et al., 1992b). Although the reasons for the
dwarf phenotype have not been unambiguously determined, it is likely that the
diversion of cytoplasmic acetyl-CoA and acetoacetyl-CoA away from the endogenous
isoprenoid and flavonoid pathways might lead to a depletion of essential
metabolites, such as sterols, which may affect growth.
Synthesis of PHB in the cytoplasm of rape leaf cells gave results very similar to
those in
Arabidopsis (Poirier, 2002). Interestingly, overexpression of the bacterial
3-ketothiolase in plants expressing the reductase and PHB synthase did not lead to
a significant increase in PHB accumulation, indicating that 3-ketothiolase activity
was probably not limiting PHB synthesis in the cytoplasm, but that other factors,
such as the low flux of acetyl-CoA, may be important.
PHB synthesis has also been demonstrated in the cytoplasm of cotton fiber
cells (John and Keller, 1996). In this approach, PHA is not produced as a source of
polyester to be extracted and used in the plastic industries, but rather as an
intracellular agent that modifies the heat exchange properties of the fiber. The
phaA,
phaB, and
phaC genes from
R. eutropha were expressed in transgenic cotton
under the control of a fiber-specific promoter (John and Keller, 1996). PHB accumulated
in the cytoplasm to 0.3% dwt of the mature fiber, a level similar to PHB
production in
A. thaliana cell cytoplasm, while no deleterious effect on fiber
development was reported.
Production of PHB has been reported in leaves of
Nicotiana tabacum through
the coexpression of the
phaB gene from
R. eutropha and the PHA synthase from
Aeromonas caviae (Nakashita
et al., 1999). Although the bacterial genes were
expressed under the strong promoter CaMV35S, expression of both proteins
was relatively low and the amount of PHB detected in leaves was only 10 µg/g
fresh weight (fwt). Inhibition of the mevalonate pathway at the level of the
3-hydroxy-3-methylglutaryl-CoA reductase led to a twofold increase in PHB
level in tobacco cell lines, indicating a link between PHB synthesis and availability
of acetyl-CoA (Suzuki
et al., 2002). Similar levels of PHB were obtained in potato
expressing the phb enzymes in the cytosol.
Synthesis of PHB in the Plastid
The relatively limited supply of acetyl-CoA in the cytosol is thought to be responsible
for the low accumulation of PHB as well as for the deleterious effects of
transgene expression on plant growth observed in many plants. In this context,
the plastid was viewed as a much better site for PHB synthesis, since this organelle
has a larger flux of carbon through acetyl-CoA required for fatty acid biosynthesis.
This is particularly true for the leucoplast of developing seeds of oil-accumulating
plants, such as
Arabidopsis and oilseed rape.
The
phaA,
phaB, and
phaC proteins from
R. eutropha were modified for plastid
targeting by addition of the transit peptide of the small subunit of the ribulose
bisphosphate carboxylase from pea (Nawrath
et al., 1994). The modified bacterial
genes were first expressed individually in
A. thaliana under the control of the
constitutive CaMV35S promoter, and later the transgenes were combined through
crossings. Transgenic plants expressing only the plastid-targeted reductase
and PHA synthase did not produce detectable PHB, providing further evidence
that plastids do not have an endogenous 3-ketothiolase activity that could support
PHB synthesis (Nawrath
et al., 1994). However, plants expressing all three bacterial
enzymes were shown to accumulate PHB inclusions exclusively in the plastids,
with some organelle having a substantial portion of their volume filled with
inclusions. The size and general appearance of these were similar to bacterial
PHA inclusions (Nawrath
et al., 1994). Interestingly, the quantity of PHB in these
plants was found to gradually increase over time, with fully expanded presenescing
leaves typically accumulating 10 times more PHB than do young expanding
leaves of the same plant. The maximal amount of PHB detected in presenescing
leaves was 10 mg/g fwt, representing ~14% dwt. In contrast to PHB synthesis in
the cytoplasm, expression of the PHB biosynthetic enzymes in the plastid was not accompanied by a large reduction in growth of these plants. However, leaf chlorosis
was observed in plants accumulating more than 3–5% dwt. These results indicated
that although the plastid can accommodate a higher production of PHB with minimal
impact on plant growth compared to the cytoplasm, there was nevertheless a
limit above which alteration in some of the chloroplast functions could be detected
(Nawrath
et al., 1994).
In contrast to the individual expression of the
R. eutropha phb genes in plants
followed by stacking through crossing, an alternative strategy was devised where
all three plastid-modified
phb genes were cloned into a single binary vector.
By this approach, a number of lines were identified which accumulated PHB
between 3% and 40% dwt (Bohmert
et al., 2000). While in a line accumulating
3% dwt most of the plastids contained some PHB inclusions, all plastids of
mesophyll cells were packed with inclusion in the line containing PHB to 40%
dwt. Interestingly, these transgenic plants showed a negative correlation between
PHB accumulation and plant growth. While plants containing 3% dwt PHB
showed only a relatively small reduction in growth, plants accumulating between
30% and 40% dwt PHB were dwarfed and produced no seeds (Bohmert
et al., 2000). As previously observed by Nawrath and colleagues, all plants producing
above 3% dwt PHB showed some chlorosis (Bohmert
et al., 2000; Nawrath
et al., 1994). Together, these experiments demonstrate that while it is possible to further
increase PHB production in plastids by using new vectors, the approach of
synthesizing PHB in the chloroplasts of shoots has its limits.
Since the production of PHA in the plastid typically requires the expression of
several enzymes, strategies devised to simplify the number of individual genes
that must be expressed could have advantages. In this respect, a novel fusion
protein composed of the 3-ketothiolase and acetoacetyl-CoA reductase from
R. eutropha was created (Kourtz
et al., 2005). This was a challenging project since
the native thiolase and reductase enzymes act as homotetramers in bacteria.
Nevertheless, one fusion protein exhibited thiolase and reductase activities in
crude extracts of recombinant
Escherichia coli that were only threefold and ninefold
less than those of the individually expressed thiolase and reductase enzymes,
respectively. Expression of the plastid-targeted fusion enzyme, along with the
PHA synthase, resulted in plants accumulating roughly half the amount of PHB
synthesized in plants expressing the individual enzymes.
As a first step to bring the technology of PHA synthesis to the field, scientists at
Monsanto have demonstrated the production of PHB in the plastids of corn leaves
and stalk, as well as in the leucoplast of developing seeds of
Brassica napus.
In those experiments, the same
R. eutropha genes modified for PHB production
in the plastids of
A. thaliana were used. Levels of PHB accumulation up to 5.7%
dwt were reported (Poirier and Gruys, 2001). Similar to results obtained in
A. thaliana, there was a progressive accumulation of PHB in corn shoots with
time, with older leaves having more polymer than younger leaves. Furthermore,
like in
A. thaliana, there was a correlation between leaf chlorosis and higher amount
of PHB (Poirier and Gruys, 2001). Perhaps one of the most striking observations
made from the experiments in corn was the fact that while the leaf mesophyll cells
showed few PHB granules, the bundle sheath cells associated with the vascular tissue were packed with granules (Poirier and Gruys, 2001).
This unequal distribution
of PHB was not due to the promoter used, since a similar pattern was seen
for plants transformed with either the CaMV35S or the chlorophyll A/B binding
protein promoters, the latter promoter being known to be a strong promoter in
mesophyll cells. Interestingly, a similar observation had been made by the same
group for
A. thaliana plants transformed with the
phb genes driven by the
CaMV35S promoter; that is, significantly more granules were found in cells
surrounding the vascular tissue and epidermal cells compared to mesophyll
cells (Poirier and Gruys, 2001). These results suggest that the availability of
plastidial acetyl-CoA for PHB synthesis may be quite different in various cell
types, perhaps due to metabolic channeling.
For the creation of the PHB biosynthetic pathway in developing seeds of
B. napus, the three modified bacterial genes
phaA,
phaB, and
phaC were put
under the control of the fatty acid hyroxylase promoter from
Lesquerella fendeleri,
enabling strong expression to the developing seed (Houmiel
et al., 1999). PHB
level up to 7.7% fwt of mature seeds was reported (Houmiel
et al., 1999). Analysis
of seeds by TEM revealed that PHB accumulated exclusively within the leucoplast
and that apparently every visible plastid contained the polymer. Seeds accumulating
nearly 8% dwt PHB appeared normal and germinated at the same rates as
nontransformed seeds (Houmiel
et al., 1999). These results demonstrate that at
least in the range of 3–8% dwt PHB, the seed leucoplast appears a better production
system than the leaf chloroplast. It is unknown at this point what is the upper
limit of PHB accumulation in seeds and at what level PHB synthesis will start
affecting the accumulation of lipids or proteins in the seed, two key factors that
have a strong impact on the viability of this approach in the biotechnological
production of PHA in oilseed crops.
Five additional crop plants have been investigated for PHA production
through expression of the PHB pathway in the plastid. Transformation of alfalfa,
tobacco, potato, and flax with the three
R. eutropha phb genes modified for plastid
targeting was shown to give transgenic plants producing PHB in their leaves to a
maximum level of 0.18%, 0.32%, 0.009%, and 0.005% dwt (Bohmert
et al., 2002;
Saruul
et al., 2002; Wróbel
et al., 2004). Although the reasons behind the low level
of PHB accumulation in these plants compared to either
Arabidopsis or corn have
not been fully elucidated, it has been demonstrated that constitutive expression of
the bacterial 3-ketothiolase leads to a large decrease in the recovery of transgenic
plants following transformation (Bohmert
et al., 2002). The use of a construct
where the bacterial 3-ketothiolase is expressed under the control of an inducible
promoter led to an increased recovery of transgenic tobacco and potato producing
PHB, although the amount of PHB produced remained relatively low at below
0.3% dwt (Bohmert
et al., 2002). Transformation of
in vitro cultured hairy roots of
sugar beet with the same three
R. eutropha genes modified for plastid targeting led
to significantly higher amount of PHB, with a maximum of 5.5% dwt (Menzel
et al., 2003). Thus, although accumulation of PHB in the plastid appears to be
problematic for several plants, the success encountered with
Arabidopsis, rape,
corn, and roots of sugar beet indicate that there is no fundamental barrier to
relatively high production of PHA in the plastids of plants.
As an alternative strategy to the transformation of the nuclear genome, transformation
of the plastid genome with the
phb gene has been examined. In theory,
plastid transformation could lead to higher level of polymer production because
of the much larger copy number of transplastome compared to the nuclear
genome. However, transformation of tobacco plastome with the
R. eutropha polycistronic
operon containing the
phbA,
phbB, and
phbC genes under the control of a
bacterial promoter or of the plastid rRNA promoter (
Prrn) has yielded plants
synthesizing only low amount of PHB (<0.1% PHB dwt) (Arai
et al., 2001, 2004;
Nakashita
et al., 2001). Expression of the
R. eutropha polycistronic operon under
the control of the plant
psbA promoter and the psbA 5´ UTR improved PHB
accumulation up to 1.7% dwt (Lö ssl
et al., 2003). In these transgenic plants, a
higher level of PHB was limited to the early stage of heterotrophic
in vitro culture
and decreased through autotrophic growth despite constant transcript levels.
PHB amounts were also found to be highly variable in different tissues of the
same plant. Furthermore, production of PHB in transplastomic tobacco was
associated with growth retardation and male sterility (Lö ssl
et al., 2003). Use of a
transformation system where the plastidial polycistronic phb operon was under
the control of an ethanol-inducible T7 RNA polymerase could solve the problem
of growth retardation and sterility, but without further improvement in the yield
of PHB (Lö ssl
et al., 2005). Although further work is required to understand the
factors limiting the stable production of PHB in transplastomic tobacco, it must be
stressed that accumulation of PHA in tobacco and potato, either in the cytoplasm
or in the plastid, has consistently been low compared to
Arabidopsis or rape.
In this context, it would be very interesting to know if the application of the
transplastome approach to
Arabidopsis and rape would give similar or higher
amount of PHB compared to nuclear transformation.
PHB synthesized in plants is not thought to be degraded, since significant
hydrolysis of PHA requires the presence of specialized bacterial enzymes, the
PHA depolymerases (Jendrossek, 2002). PHA in plants is thus viewed as a final
and largely unrecyclable carbon sink. This opens several interesting questions
about how transgenic plants accumulating PHA can cope with a new carbon
sink. For example, how does PHB synthesis in the plastids affect carbon flow to
other compounds synthesized in the organelle, such as starch and fatty acids?
How does the plant adjust, at the metabolic and genetic levels, to accommodate for
the synthesis of this new sink? Why are plants producing high amount of PHB
affected in their growth? Clearly, the tools of genomics, proteomics, and metabolic
profiling could provide interesting answers to these questions and give general
insights on plant biochemistry that would go well beyondPHAsynthesis in plants.
In a first small-scale study of metabolite profiling, over 60 metabolites were
measured in transgenic
A. thaliana lines producing high amount of PHB (Bohmert
et al., 2000). Surprisingly, no changes in fatty acids were observed. There was,
however, a correlation between an increase in PHB with a decrease in levels of
isocitrate and fumarate, indicating a reduction in tricarboxylic acid cycle activity,
leading perhaps to a reduction in pools of acetyl-CoA that may result in growth
retardation. There was also a positive correlation between PHB accumulation and
levels of several sugars such as mannitol, glucose, fructose, and sucrose. Together, these data indicate that a high amount of accumulation of PHB in chloroplasts has
a negative and complex effect on plant metabolism that go beyond the chloroplast.
At the gene expression level, no correlation could be found between level of
expression of the three
phb genes and PHB accumulation, leaving unresolved
the question of what limits PHB synthesis in the plastids.
Synthesis of PHB in the Peroxisome
Acetyl-CoA is found not only in the cytoplasm and plastids but also in the
mitochondria and peroxisomes, being primarily implicated in these organelles
in the tricarboxylic acid and β-oxidation cycles, respectively. Although no conclusive
demonstration of PHB in plant mitochondria has been reported, synthesis of
PHB in the peroxisome was described in transgenic Black Mexican sweet corn
suspension cell cultures (Hahn
et al., 1999). In these experiments, the
phaA,
phaB,
and
phaC genes from
R. eutropha were modified in order to add a peroxisomal
targeting signal at the carboxy terminal end of each protein. Biolistic transformation
of maize suspension culture with a mixture of all three genes led to the
isolation of transformants expressing all three enzyme activities and accumulating
PHB up to 2% dwt (Hahn
et al., 1999). As no transgenic plants have been
obtained from these transformed cells, it is difficult at this point to evaluate the
potential effects of PHB synthesis in peroxisome on growth and metabolism.






