Medium-Chain-Length Polyhydroxyalkanaote
MCL-PHAs are typically described as elastomers, although their actual physical
properties are very diverse, ranging from soft plastic to glue and rubber, and
are primarilydependent on themonomer composition (deKoning, 1995).Monomers
present in MCL-PHA may contain a wide spectrumof functional groups, including
unsaturated bonds and halogenated groups (Steinbüchel and Valentin, 1995).
There are two main routes for the synthesis of MCL-PHA in bacteria (Fig. 8.6)
(Steinbü chel and Fü chtenbusch, 1998; Steinbü chel and Hein, 2001). The first is the synthesis of PHA using intermediates of fatty acid β-oxidation. This pathway is
found in several bacteria, such as
Pseudomonas oleovorans and
Pseudomonas fragii,
which can synthesize MCL-PHA from either alkanoic acids or fatty acids. In these
bacteria, the monomer composition of the PHA produced is directly influenced by
the carbon source added to the growth media. Typically, the PHA is composed of
monomers that are 2n (n ≥ 0) carbons shorter than the substrates added to the
media. For example, growth of P.
oleovorans on octanoate (C
8) generates a PHA
copolymer containing C
8 and C
6 monomers, whereas growth on dodecanoate
(C
12) generates a PHA containing C
12, C
10, C
8, and C
6 monomers (Lageveen
et al., 1995).
Alkanoic acids present in the media are transported into the cell where they are first converted toCoAesters before being directed to the β-oxidation
pathway where a number of 3-hydroxyacyl-CoA intermediates can be
generated. Since the PHA synthase accepts only the R-isomer of 3-hydroxyacyl-
CoA and the bacterial b-oxidation of saturated fatty acids generates only the
S-isomer of 3-hydroxyacyl-CoA, bacteria must have enzymes capable of generating
R-3-hydroxyacyl-CoA. One potential enzyme is a 3-hydroxyacyl-CoA
epimerase, mediating the reversible conversion of the S- and R-isomers of
3-hydroxyacyl-CoA, although no protein or gene encoding such activity has
yet been unambiguously identified (Yang
et al., 1986). In contrast, monofunctional
enoyl-CoA hydratase II enzymes, converting directly enoyl-CoA
to R-3-hydroxyacyl-CoA, have been identified in several bacteria, including
Aeromonas caviae (Fukui
et al., 1998; Reiser
et al., 2000; Tsuge
et al., 2000). Finally,
it is speculated that a 3-ketoacyl-CoA reductase that could specifically generate
R-3-hydroxyacyl-CoA may exist in bacteria, although such an enzyme has not
yet been unambiguously identified. It has, however, been shown that the
enzyme 3-ketoacyl-acyl carrier protein (ACP) reductase, participating normally
in the fatty acid biosynthetic pathway, may also act on 3-ketoacyl-CoA to
generate R-3-hydroxyacyl-CoA, and thus contribute to MCL-PHA synthesis
(Taguchi
et al., 1999).
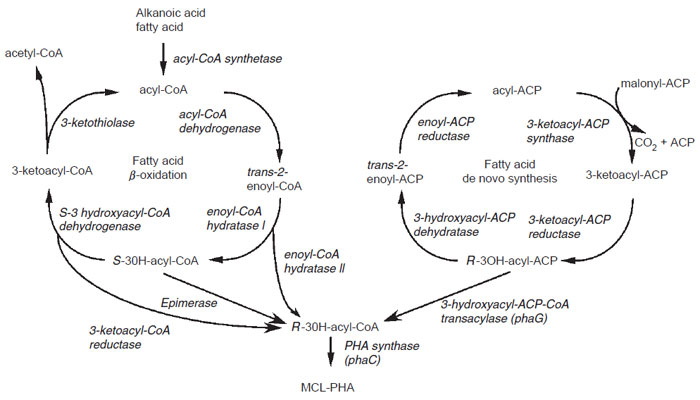 |
| FIGURE 8.6 Pathways for MCL-PHA synthesis. Synthesis of MCL-PHA in bacteria can be
accomplished either through the use of intermediates of the fatty acid β-oxidation cycle
(left) or of the de novo fatty acid biosynthetic pathway (right). |
The second route for MCL-PHA in bacteria is through the use of intermediates
of fatty acid biosynthesis (Fig. 8.6). This pathway is also found in numerous
Pseudomonads. In contrast to P.
oleovorans and P.
fragii, which can synthesize
MCL-PHA only from related alkanoic acids present in the growth media,
Pseudomonas aeruginosa and
Pseudomonas putida can synthesize a similar type of
MCL-PHA when grown on unrelated substrates, such as glucose (Huijberts
et al., 1992; Steinbüchel and Lü tke-Eversloh, 2003). In these bacteria,MCL-PHAis formed
from the 3-hydroxyacyl-ACP intermediates of the de
novo fatty acid biosynthetic
pathway.
phaG is a key enzyme in this pathway, having a 3-hydroxyacyl-CoAACP
transferase activity responsible for converting the R-3-hydroxyacyl-ACP
intermediate of the fatty acid biosynthetic pathway to R-3-hydroxyacyl-CoA, the
substrate for the PHA synthase (Rehm
et al., 1998).
Synthesis of MCL-PHA in Plants
The first approach used to synthesize MCL-PHA in plants was to divert the
3-hydroxyacyl-CoA intermediates of the b-oxidation of endogenous fatty acids.
Since in plants b-oxidation occurs in the peroxisomes, PHA biosynthetic proteins needed to be targeted to this organelle. The
phaC1 synthase from P. aeruginosa was
thus modified at the carboxy end by the addition of peroxisomal targeting signal.
The modified
phaC1 gene was expressed under the control of the CaMV35S
promoter and transformed into
A. thaliana (Mittendorf
et al., 1998). Appropriate
targeting of the PHA synthase in plant peroxisomes was demonstrated by
immunolocalization. TEM also showed the presence of typical PHA inclusions
within the peroxisomes. The monomer composition of the MCL-PHA produced in
plants reflected well the broad substrate specificity of the PHA synthase of P.
aeruginosa. Thus, peroxisomal PHA was composed of over 14 different monomers,
including saturated and unsaturated monomers ranging from 6 to 16 carbons
(Mittendorf
et al., 1998). The majority of 3-hydroxyacids found in plant MCL-PHA
could be clearly linked to the corresponding 3-hydroxyacyl-CoA generated by the
b-oxidation of saturated and unsaturated fatty acids.
The production of
peroxisomal MCL-PHA was relatively low, with a maximal level of 0.4% dwt in
7-day-old germinating seedlings. In leaves, PHA level decreased to ~0.02% dwt.
Interestingly, a two- to threefold increase in PHA was observed during leaf
senescence. These data support the link between β-oxidation and PHA synthesis,
since this pathway, in association with the glyoxylate cycle, is most active during
germination and senescence where they are involved in the conversion of fatty
acids to carbohydrates. In contrast to PHB synthesis in the cytoplasm and plastid,
no negative effects of peroxisomal MCL-PHA accumulation on plant growth or
seed germination were observed (Mittendorf
et al., 1998).
Similar to the PHA synthase from
R. eutropha, the PHA synthase of P.
aeruginosa is thought to accept only the R-isomer of 3-hydroxyacyl-CoAs. The wide
range of monomers found in plant MCL-PHA suggests that, as with bacteria,
plants also have enzymes capable of converting the β-oxidation intermediates
S-3-hydroxyacyl-CoA to the R-isomer. Such enzymes could be either the
3-hydroxyacyl-CoA epimerase present on the plant MFP or an enoyl-CoA hydratase
II activity that is specific for the generation of R-3-hydroxyacyl-CoA
from
trans-2-enoyl-CoA.
A third route for the synthesis of a narrow range of
R-3-hydroxyacyl-CoA is the hydration of
cis-2-enoyl-CoA by the enoyl-CoA
hydratase I activity of the MFP. The substrate
cis-2-enoyl-CoA is derived from
the β-oxidation of unsaturated fatty acids having a cis double bond at an even
position, such as that found in linoleic and linolenic acids (Poirier, 2002).
Growth of transgenic plants in liquid media supplemented with detergents
containing various fatty acids was used to study how to influence the quantity
and monomer composition of PHA produced from β-oxidation. Addition of
external fatty acids to plants resulted in both an increased accumulation of
MCL-PHA and a shift in the monomer composition that reflected the intermediates
generated by the β-oxidation of the external fatty acids (Mittendorf
et al., 1999).
For example, addition of the detergent polyoxyethylenesorbitan esterified
to lauric acid (Tween-20) to the media resulted in an eight- to tenfold increase in
the amount of PHA synthesized in 14-day-old plants compared to plants growing
in the same media without detergent. The monomer composition of the MCLPHA
synthesized media containing Tween-20 showed a large increase in the
proportion of saturated even-chain monomers with ≤12 carbons, and a corresponding decrease in the proportion of all unsaturated monomers. This shift
in monomer composition is accounted by the fact that β-oxidation of lauric acid,
a 12 carbon saturated fatty acid, gives saturated 3-hydroxyacyl-CoA intermediates
of 12 carbons and lower. Further experiments have shown that addition of
either tride-, tridecenoic acid (C13:1 D12), or 8-methyl-nonanoic acid in the plant
growth media resulted in the production of MCL-PHA containing mainly
saturated odd-chain, unsaturated odd-chain, or branched-chain 3-hydroxyacid
monomers, respectively (Mittendorf
et al., 1999). These results demonstrated
that the plant b-oxidation cycle was capable of generating a large spectrum of
monomers that can be included in MCL-PHA even from fatty acids that are not
present in significant quantities in plants. Furthermore, ‘‘feeding’’ experiments
with these unusual fatty acids demonstrated that all 3-hydroxyacids between
6 and 16 carbons that could be generated by the β-oxidation cycle (via the
3-hydroxyacyl-CoA intermediate) were found in the MCL-PHA. These results
supported the concept that the monomer composition of PHA could be used as
a tool to study the degradation pathway of fatty acids, including unsaturated
fatty acids.
As an alternative to the addition of external fatty acids, modulation of the
monomer composition of MCL-PHA synthesized in peroxisomes was also
achieved by modifying the endogenous fatty acid biosynthetic pathway
(Mittendorf
et al., 1999). The first example of this approach was the expression
of the peroxisomal PHA synthase in a mutant of
A. thaliana deficient in the
synthesis of triunsaturated fatty acids. MCL-PHA produced from this mutant
was almost completely deficient in all 3-hydroxyacids derived from the degradation
of triunsaturated fatty acids, including triunsaturated monomers (Mittendorf
et al., 1999). Since numerous fatty acids desaturases have now been cloned and
expressed in transgenic plants to control the number and position of unsaturated
bonds in fatty acids, this approach could be extended to further modulate the
proportion of a number of 3-hydroxyacid monomers in PHAs.
The second approach used to influence the quantity and monomer composition
of MCL-PHA was the coexpression of a medium-chain thioesterase in the
plastid with a PHA synthase in the peroxisome. Studies on transgenic plants
expressing a laurate acyl-ACP thioesterase in the plastid of either leaves or
seeds of rape revealed the presence of a futile cycling of lauric acid whereas a
substantial portion of the unusual fatty acid was degraded through peroxisomal
β-oxidation instead of accumulating in lipids (Eccleston and Ohlrogge, 1998;
Eccleston
et al., 1996).
These studies on lauric acid-producing rapeseed indicated
that expression of a thioesterase might be a way of increasing the carbon flux
toward β-oxidation and peroxisomal PHA biosynthesis. This hypothesis was
tested in
A. thaliana by combining the constitutive expression of the peroxisomal
PHA synthase with the caproyl-ACP thioesterase from
Cuphea lanceolata in the
plastid (Mittendorf
et al., 1999). Expression of both enzymes led to a seven- to
eightfold increase in the amount of MCL-PHA synthesized in plant shoots as
compared to transgenics expressing only the PHA synthase. Furthermore, the
composition of the MCL-PHA in the thioesterase/PHA synthase double transgenic
plant was shifted toward saturated 3-hydroxyacid monomers containing 10 or fewer carbons. This shift is in agreement with an increase in the flux of
decanoic acid toward b-oxidation triggered by the expression of the caproyl-ACP
thioesterase (Mittendorf
et al., 1999). Interestingly, constitutive expression of the
related lauroyl-ACP thioesterase in
A. thaliana was shown not to lead to an
increase in the genes or enzymes involved in β-oxidation (Hooks
et al., 1999).
The relation between fatty acid futile cycling and peroxisomal PHA synthesis
was further extended to the developing seeds (Poirier
et al., 1999). Synthesis of
MCL-PHA has been demonstrated in seeds of
A. thaliana by expressing the
peroxisomal PHA synthase gene under the control of the seed-specific napin
promoter. In such transgenic plants, MCL-PHAs accumulated to 0.006% dwt in
mature seeds and the monomer composition was relatively similar to the PHA
synthesized in germinating seedlings.
Expression of both the PHA synthase and
caproyl-ACP thioesterase in the leucoplasts of developing seeds resulted in a
nearly 20-fold increase in seed PHA, reaching 0.1% dwt in mature seeds. Furthermore,
as found with the expression of these two enzymes in whole plants, coexpression
in seeds resulted in a large increase in the proportion of 3-hydroxyacid
monomers containing 10 or fewer carbons in PHA. These data clearly indicate that
even though expression of the caproyl-ACP thioesterase in seeds leads to the
accumulation of medium-chain fatty acids in triacylglycerides, there are still a
significant proportion of these fatty acids that are channeled toward b-oxidation.
This flux toward the β-oxidation cycle is thought to be quite significant, considering
that there is only a fourfold difference between the maximal amount of PHA
synthesized in germinating seedlings (0.4% dwt), where β-oxidation is thought to
be maximal, and the PHA synthesized in the developing seeds expressing the
thioesterase (0.1% dwt), where metabolism should be mainly devoted to the
synthesis of fatty acid instead of degradation.
Synthesis of MCL-PHAin the peroxisomes of developing seeds has alsodemonstrated
the presence of an increased cycling of fatty acids toward β-oxidation
in plants deficient in the enzyme diacylglycerol acyltransferase (DAGAT) (Poirier
et al., 1999). The
tag1 mutant of
A. thaliana was shown to be deficient in DAGAT
activity in developing seeds, resulting in a decreased accumulation of triacylglycerides
and corresponding increase in diacylglycerides and free fatty acids in
mature seeds (Katavic
et al., 1995). It was hypothesized that the imbalance created
between the capacity of the plastid to synthesize fatty acids and the capacity of the
lipid biosynthetic machinery of the ER to include these fatty acids into triacylglycerides
might have two basic consequences: either fatty acid biosynthesis would
be reduced (feedback inhibited) in order to match it with triacylglyceride biosynthesis
or excess fatty acids that cannot be included in triacylglycerides would be
channeled toward β-oxidation. Expression of the peroxisomal PHAsynthase in the
tag1 mutant resulted in a tenfold increase in the amount of MCL-PHA accumulating
in mature seeds compared to expression of the transgene in wild-type plants
(Poirier
et al., 1999). Although these results do not address whether fatty acid
biosynthesis is decreased in the
tag1 mutant, they nevertheless clearly indicate
that a decrease in triacylglyceride biosynthesis results in an increase in the flux of
fatty acids toward β-oxidation. Thus, carbon flux to the β-oxidation cycle can be
modulated to a great extent and appears to play an important role in lipid homeostasis in plants even in tissues that are primarily devoted to lipid biosynthesis,
such as the developing seeds.
Analysis of futile cycling of fatty acids in developing seeds has been extended
to transgenic plants accumulating the unusual fatty acids, ricinoleic acid and
vernolic acid (Moire
et al., 2004).
A. thaliana expressing either the
Ricinus communis oleate 12-hydroxylase or the
Crepis palaestina linoleate 12-epoxygenase under the
control of the napin promoter was shown to accumulate approximately twofold
more MCL-PHA in developing seeds compared to control. Although relatively
small compared to the increase in PHA observed in transgenic plants expressing
the C.
lanceolata caproyl-ACP thioesterase, the twofold increase in MCL-PHA was
quite significant considering that the steady level of either hydroxy or epoxy fatty
acids accumulated in transgenic seeds represented only 6.3 mol% or 3.1 mol%,
respectively. Thus, clearly, a larger proportion of unusual fatty acids were
being degraded via peroxisomal β-oxidation in developing seeds compared to
the common fatty acids. Interestingly, microarray analysis of nearly 200 genes
involved in fatty acid biosynthesis and degradation, including the genes
encoding enzymes of the β-oxidation cycle, revealed no changes in gene expression
in transgenic developing seeds expressing either C. l
anceolata caproyl-ACP
thioesterase, R.
communis oleate 12-hydroxylase, or C.
palaestina linoleate
12-epoxygenase (Moire
et al., 2004). These results indicated that analysis of peroxisomal
PHA is a better indicator of the flux of fatty acid through b-oxidation than
the expression profile of genes involved in lipid metabolism.
Synthesis of a ‘‘hybrid’’ PHA copolymer has been reported in
A. thaliana expressing a PHA synthase from A.
caviae modified at the carboxy terminal end
for targeting to the peroxisome (Arai
et al., 2002). Expression of this PHA synthase
under the control of the CaMV35S promoter leads to the accumulation of a PHA
containing even-chain and odd-chain monomers ranging from 4 to 6 carbons. The
maximal amount of PHA accumulated in leaves and seeds was 0.04% and 0.0032%
dwt, respectively. Growth of transgenic plants in media containing Tween-20
increased the total amount of PHA synthesized without affecting appreciably
the monomer composition (Arai
et al., 2002). The incorporation of ~25 mol% of
3-hydroxyvalerate into PHA raises the interesting question of the source of the
odd-chain monomer. Although odd-chain monomers have been detected in MCLPHA
synthesized from the expression of the P.
aeruginosa PHA synthase in the
peroxisome, the amount of odd-chain monomers was very low (<1 mol%). It is
possible that an α-oxidation pathway could generate odd-chain intermediate from
even-chain fatty acids and that this pathway is more active toward shorter chain
intermediates (i.e., 6 carbon fatty acids). Although a gene involved in α-oxidation
has been identified, the corresponding protein has not been linked to the peroxisome
(Hamberg
et al., 1999). Thus, despite evidence of a complete α-oxidation
pathway in plants, the link between this pathway and the peroxisome needs to be
established. PHA thus offers potentially a unique handle to study α-oxidation in
plants.
In the bacterial pathway of MCL-PHA synthesis from intermediates of fatty
acid biosynthesis, the enzyme
phaG plays a key role, catalyzing the conversion of
R-3-hydroxyacyl-ACP to R-3-hydroxyacyl-CoA, the latter being the substrate for the PHA synthase (Rehm
et al., 1998). The identification and cloning of the
P.
putida phaG gene opened the possibility of synthesizing PHA copolymers in
the plastids of plants from intermediates of fatty acid biosynthesis. Unfortunately,
constitutive expression in the plastid of
A. thaliana of only the
phaG enzyme led to
a marked deleterious effect on plant growth, the plants being dwarfed with
crinkly leaves and the seed set being strongly reduced (V. Mittendorf, unpublished
results). The reason for this phenotype is not known but is thought to be
perhaps due to interference of the transacylase with fatty acid biosynthesis. If this
is the case, it would be interesting to know why this does not occur in bacteria
expressing
phaG. Coexpression in the plastid of the P.
aeruginosa PHA synthase
along with
phaG did not conclusively lead to PHA accumulation in
Arabidopsis (V. Mittendorf, unpublished results). Analogous experiments in potato led to
similar conclusions, although evidence for the synthesis of a very small amount
of a hydrophobic polymer that could be MCL-PHAs was provided (Romano
et al., 2005).
Thus, despite the obvious advantages of the plastid as a location for the
production ofPHB and P(HB-HV), the synthesis in this organelle of
phaCopolymer
using fatty acid biosynthetic intermediates appears problematic at present.
The synthesis of MCL-PHA in potato cell lines has been demonstrated through
expression of the PHA synthase from P.
oleovorans in the cytoplasm (Romano
et al., 2003). PHA could be detected only after ‘‘feeding’’ the cell lines with
3-hydroxyoctanoic acid, with the PHA containing only the 8 carbon monomer.
These results indicate that while no endogenous 3-hydroxyacyl-CoA could be
detected in the cytoplasm, an acyl-CoA synthetase activity capable of converting
3-hydroxyoctanoic acid (that originally comes from the external media) to the
corresponding 3-hydroxyacyl-CoA was present. The amount of PHA detected
reached up to 1% dwt.





