Engineering Post-Rubisco Reactions
Flux control analysis indicated SBPase as the most likely rate-limiting step for regeneration of RuBP in the PCR cycle (Robinson and Walker, 1981; see Section 2.2). Furthermore, the two phosphatases FBPase and SBPase, as well as PRK, are light-regulated enzymes that avoid futile reactions in the dark. Regulation is exerted through the redox reaction of two SH-groups in these proteins (Buchanan, 1991). The SH-groups are also targets of hydrogen peroxide under oxidative stress that affects redox homeostasis (Shikanai et al., 1998).
In contrast to the plant PCR cycle, cyanobacterial and green algal PCR pathways are insensitive to oxidation by H2O2 and are not subject to light/dark regulation (Tamoi et al., 1998). This is because the enzyme involved in the rate-limiting step of these microorganisms lacks the functional redox-responding SH-groups (Tamoi et al., 1996a,b, 2001). While the plant and algal PCR cycles include FBPase and SBPase as separate entities, both metabolic steps are catalyzed by a single enzyme, FBP/SBPase, in the PCR cycle of Synchococcus (Tamoi et al., 1996b). The bifunctional enzyme lacks regulatory SH-groups. The gene for the cyanobacterial enzyme fused to a RuBisCO small subunit transit peptide has been introduced into tobacco (Miyagawa et al., 2001; Tamoi et al., 2005). The transformant created in this experiment revealed improved photosynthetic performance: transformed plants showed a 2.3-fold increase in chloroplast FBPase and SBP activities relative to wild type, accompanied by an increase in CO2-fixation rate and dry matter to 125% and 150%, respectively, of the wild type (Fig. 4.3). The photosynthetic rates realized in these transformants may be the maximum attainable for C3 photosynthesis because C3 photosynthesis enters a Pi-limited state at such high CO2-fixation rates (see section 2.1).
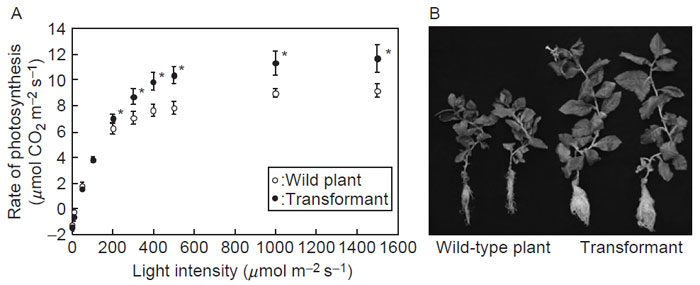 |
| FIGURE 4.3 Phenotypes of the wild-type tobacco plant and the transformant expressing cyanobacterial FBPase/SBPase in chloroplasts. (A) Effect of increasing light irradiance on the net CO2 assimilation at 360 ppm of CO2, 25°C, and 60% relative humidity. The CO2 assimilation rate was measured using the fourth leaves down from the top of plant, after 12 weeks of culture. (B) Photographs of the wild plant and the transformant after 18 weeks of culture in 360-ppm CO2 at 400 mmol m-2 s-1. |




