Minor nutrients
Content
Minor nutrients, also known as trace elements or micro-elements, are present in plants in very small quantities, but are just as essential for healthy growth as major elements. However, they can be toxic to plants if too abundant. This means that rectifying deficiencies with soluble salts has to be undertaken carefully. Deficiencies Simple deficiencies are those in which too little of the nutrient is present in the growing medium. Most soils have adequate reserves of trace elements, so simple deficiencies in them are uncommon, especially if replenished with bulky organic matter. Sandy soils tend to have low reserves and so too have several organic soils from which trace elements have been leached. In horticulture simple deficiencies of trace elements are mainly associated with growing in soil-less composts which require careful supplementation. Induced deficiencies are those in which sufficient nutrients are present, but other factors, such as soil pH or ion antagonism, interfere with plant nutrient availability: On mineral soils boron, copper, zinc, iron and manganese become less available in alkaline soils, whereas molybdenum availability is reduced severely in soils with pH levels below 5.5, as shown in Figure 20.4. Trace element problems are aggravated in dry soils or where waterlogging, root pathogens or poor soil structure reduces root activity. Irondeficiency is induced by the presence of large quantities of calcium and this ‘lime induced’ chlorosis (yellowing) occurs on over-limed soils and calcareous soils. The natural flora of chalk and limestone areas is calcicoles. Other plants grown in such conditions usually have a typically yellow appearance. Deficiencies can also be induced by high levels of copper, manganese, zinc and phosphorus. Top fruit and soft fruit are particularly susceptible, as well as crops grown in complete nutrient solutions. The problem is overcome by using iron chelates. Boron deficiency tends to occur when pH is above 6.8. It is readily leached from peat. Crops grown in peat are particularly susceptible when pH levels rise (Figure 20.4). Boron can be applied to soils before seed sowing in the form of borax or ‘Solubor’. Manganese deficiency is more frequent on organic and sandy soils of high pH. Plant uptake can be reduced by high potassium, iron, copper and zinc levels. Manganese availability is greatly increased at low pH and can reach toxic levels which most commonly occur after steam sterilization of acid, manganese-rich soils. High phosphorus levels can be used to reduce the uptake of manganese in these circumstances. Copper deficiency usually occurs on peat and sands, notably reclaimed heath-land, and in thin organic soils over chalk. High rates of nitrogen can accentuate the problem. Soils can be treated with copper sulphate or plants can be sprayed with copper oxychloride. Zincdeficiencies are not common and are usually associated with high pH. Molybdenum deficiency occurs in most soil types at a low pH (see Figure 20.4). Availability becomes much reduced below pH 5.5, especially in the presence of high manganese levels. Cauliflowers are particularly susceptible and soils are limed to solve the problem. Sodium or ammonium molybdate can be added to growing media or liquid feeds where molybdenum supplies are inadequate. Basic chemistry Some knowledge of chemistry can give insights into biology and thus horticulture. Chemistry deals with the reactions that occur when different chemical substances are brought into contact with each other. Elements: All substances are made up of elements. There are 92 stable elements, such as oxygen and hydrogen. Each element occurs in nature as atoms.
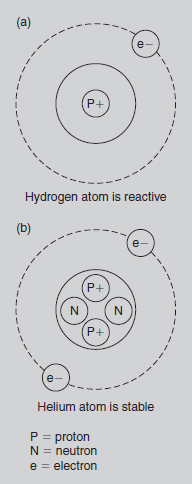
At its simplest, the chemistry of an element may be considered in terms of the group of electrons’ ‘quest for stability’. The first (inner) orbital is described as stable when it has either zero or two electrons. Outer orbitals are considered stable when they have zero or eight electrons. Four examples are used to illustrate this principle.
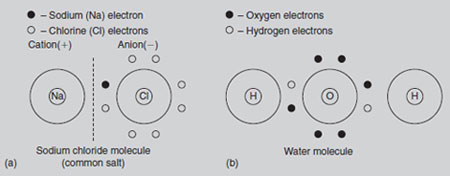
Ionic compounds: Ionic compounds, such as salts, are made up of charged atoms (ions). In the case of common salt (sodium chloride), the sodium and chlorine atoms react together (see Figure 21.7). The metallic sodium atom gives away its negative electron, thus becoming a positively charged ion called a cation: At the same time, the chlorine atom receives the negative electron from the sodium, thus becoming a negatively charged ion called an anion: Ionic substances, such as sodium chloride and many fertilizers, allow an electric current to pass through them when they are dissolved in water. Horticulturists are able to assess the strength of dissolved fertilizers in soils and composts by measuring this current (conductivity). Compound ions: Some anions occur in a compound form. Carbonate (CO32-), Nitrate (NO3-), Nitrite (NO2-), phosphate (PO43-) and sulphate (SO42-) are some examples. One cation, ammonium (NH4+) is commonly found in fertilizers. In contrast to ionic compounds, the element carbon shares electrons with the element it reacts with to produce molecules, many of which are very large (see carbon chemistry). Oxygen is a gas like hydrogen but differs from it in several ways. It is much heavier, having 8 protons and 8 neutrons (and 8 electrons) and an atomic weight of 16. Its inner electron orbital is stable, with 2 electrons, leaving six electrons in the second orbital. Oxygen therefore needs to receive two electrons to become stable. It can be seen that oxygen’s combining power is twice that of hydrogen. Oxygen is thus said to have a valency
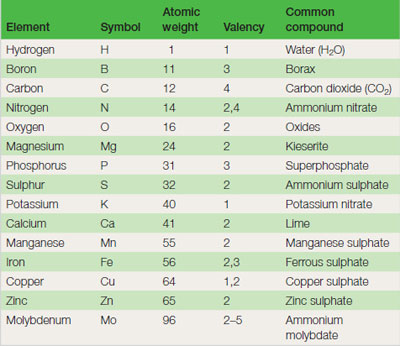
Horticultural plants require 15 elements for their growth. Table 21.1 lists the atomic weights and valency for each of the elements. Molecular weight: Examples of simple molecules are given in the ‘common compounds’ section of Table 21.1 . The molecular weight of a substance can be calculated by adding the individual atomic weights of the elements within it. For example, the molecular weight of ammonium nitrate (NH4NO3) is 14 + 1 + 1 + 1 + 1 + 14 + 16 + 16 + 16 = 80. Note that there are two nitrogen atoms in the compound, contributing 28 parts out of the total of 80 i.e. 35 per cent. Fertilizers of this type are not quite as pure and typically contain 33 per cent nitrogen. |
||||||||||||||||||||||||