Sources of nutrients
Content
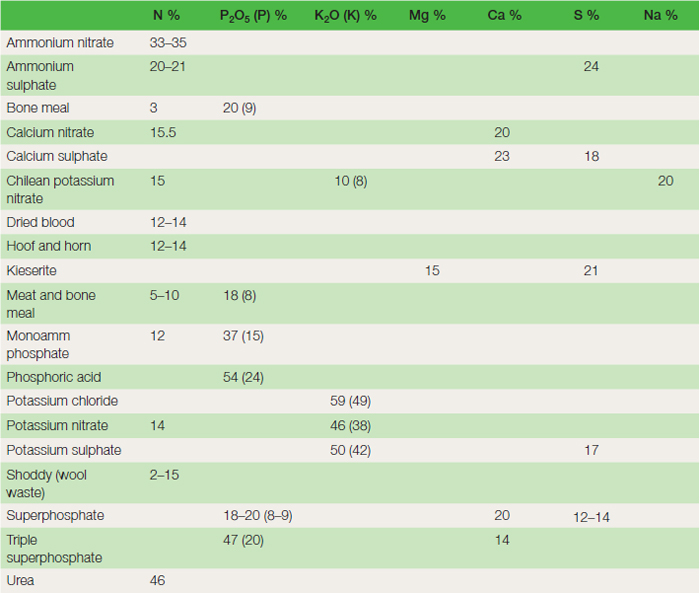
Nutrients are supplied naturally from the decomposition of organic matter and are released as clay weathers in the soil. In horticulture, additional supplies are made by the use of organic and inorganic fertilizers, bulky organic matter and through green manuring. Fertilizers Straight fertilizers are those that supply only one of the major nutrients: nitrogen, phosphorus, potassium or magnesium (see Table 21.2). The amount of nutrient in the fertilizer is expressed as a percentage. Nitrogen fertilizers are described in terms of percentage of the element nitrogen in the fertilizer, i.e. per cent N. Phosphate fertilizers have been described in terms of the equivalent amount of phosphoric oxide, i.e. per cent P2O5, or increasingly as percentage phosphorus, per cent P. Likewise potash fertilizers, i.e. per cent K2O, or percentage potassium, per cent K. Magnesium fertilizers are described in terms of per cent of Mg. The percentage figures clearly show the quantities of nutrient in each 100 kg of fertilizer. Compound fertilizers are those that supply two or more of the nutrients nitrogen, phosphorus and potassium. The nutrient content expressed as for straight fertilizers is, by convention, written on the bag in the order nitrogen, phosphorus and potassium. For example, 20–10–10 denotes 20 per cent N, 10 per cent P2O5 and 10 per cent K2O.
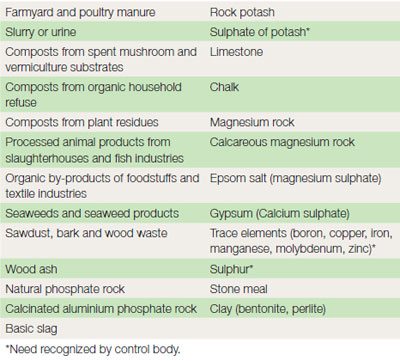
Fertilizer regulations require that further details of trace elements, pesticide content and phosphorus solubility should appear where applicable on the invoice. Fertilizers and manures are available in many different forms. Generally the term organic implies that the fertilizer is derived from living organisms, whereas inorganic fertilizers are those derived from non-living material. However, in the context of organic growing it is necessary to look at specific requirements of the regulations ( Table 21.3). Application methods Fertilizers are applied in several different ways.
Formulations Quick-acting fertilizers contain nutrients in a form which plant roots can take up, and dissolve as soon as they come in contact with water, e.g. ammonium nitrate and potassium chloride. Many are obtainable as powders or crystals. These are difficult to spread or place evenly, but several are formulated this way to help in the preparation of liquid feeds. For this purpose they must be readily soluble and free of impurities that might lead to blockages in the feed lines. Some of the less soluble fertilizers, as well as lime, are spread in a finely divided form for maximum effect on soil. One of the major problems with many of the fertilizers is their hygroscopic nature, i.e. they pick up water from the atmosphere and create storage and distribution problems. Powdered forms, in particular, go sticky as they take up water then ‘ cake’ (form hard lumps) as they dry. Fertilizers formulated as granules are more satisfactory for accurate placement or broadcasting. They flow better, can be metered, and are thrown more accurately. Prilled fertilizers represent an improvement on granules because of their uniform spherical shape. Slow release fertilizers are those in which a large proportion of the nutrient is released slowly. Several of these fertilizers, such as rock phosphate, are insoluble or only slightly soluble and the nutrients are released only after many months, even years. Micro-organisms break down organic products and the rate at which nutrients become available depends on their activity (see bacteria). Some slow release artificial fertilizers, such as those based on urea formaldehyde, dissolve slowly in the soil solution whilst others are formulated in such a way that the soluble fertilizer they contain diffuses slowly through a resin coat, e.g. Osmocote, or sulphur coating. Some of these slow-release fertilizers have been formulated in such a way as to release nutrients at a rate that matches a plant’s uptake and as such are sometimes referred to as controlledrelease fertilizer. Frits, made from fine glass powders containing nutrient elements, are used either to release soluble materials slowly or to overcome the trace element problem caused by the narrow limits between deficiency and toxicity (see trace elements). Frits ease the difficulties experienced in mixing tiny amounts evenly through large volumes of compost. Ion-exchange resins release their nutrients by exchange with cations in the surrounding water. These resins help to overcome the problems of high salt concentration and leaching of nutrients from growing media based on inert materials (see aggregate culture). Some plant nutrients are formed as chelates to maintain availability in extreme conditions where the mineral salt is ‘locked up’. There are many different chelating or sequestrating agents selected, for each element to be protected and for each unavailability problem. Iron is chelated with EDDHA to form the product Chel 138 or Sequestrine 138 which releases the element in all soils including those with a high pH (see iron deficiency). EDTA, effective where there are high levels of copper, zinc and manganese, is used to chelate iron to be applied in foliar sprays. Bulky organic matter Compost, straw, farmyard manure, bark and peat are important in horticulture as a means of maintaining organic matter and humus levels. However, they tend to be low in nutrients and some, such as straw and bark, lead to locking up of nutrients (see C:N ratio. They can be evaluated on the basis of their effect on the physical properties of soil and their small, nutrient content. Green manures Green manuring is the practice of growing plants primarily to develop and maintain soil structure and fertility. The plants used are typically agricultural crops that cover the ground quickly and yield a large amount of leaf to incorporate. The seeds are normally broadcast sown in the autumn when there are no other overwintering plants. The plants are then dug or ploughed in when the land is needed again. |
||||||||||||||||||||||