Molecular Biology of Plant Pathways / Metabolic Engineering of the Content and Fatty Acid Composition of Vegetable Oils
TAG Synthesis
TAG synthesis is a complex, multistep pathway involving multiple cellular
compartments (Fig. 7.3). Plastids, whether the chloroplasts of photosynthetic
organs or the tiny proplastids of typical oilseeds, build 2-carbon units into fatty
acids with up to 18 carbons and 1 double bond. Two of these acyl units are then
esterified to glycerol-3-phosphate, producing phosphatidic acid. The endoplasmic
reticulum (ER) is the major site of phosphatidic acid synthesis for TAG; however,
plastids likewise generate phosphatidic acid, and flow of glycerolipid backbones
from the plastids into storage oils has been observed. Fatty acids ultimately
incorporated into TAG can undergo further desaturation, elongation, or other
modifications, often while the acyl units are esterified to phosphatidylcholine
(PC) or coenzyme A. Finally, phosphatidic acid is dephosphorylated at the ER
to form diacylglycerol (DAG), and a diacylglycerol acyltransferase (DGAT) adds
the final fatty acid, forming TAG that is sequestered from the ER into lipid bodies
for storage. Alternative mechanisms for transfer of fatty acids to TAG are also
possible, as will be discussed below.
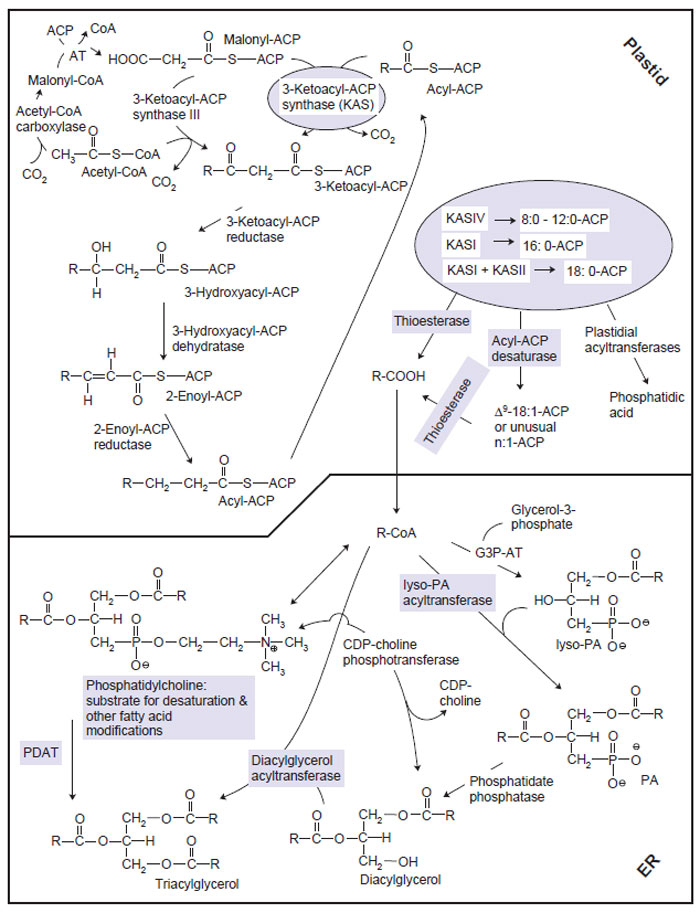 |
| FIGURE 7.3 Triacylglycerol (TAG) synthesis, highlighting points in the pathway at which genetic
engineering and/or mutagenesis have been used to modify fatty acid composition of the resulting
oil. The upper left portion of the diagram shows synthesis of malonyl-CoA by ACCase, and
the cyclic nature of the reactions catalyzed by fatty acyl synthase (FAS). FAS is composed of
malonyl-CoA:malonyl-ACP acyltransferase (AT), 3-ketoacyl-ACP synthase (KAS), 3-ketoacyl-ACP
reductase, 3-hydroxyacyl-ACP dehydratase, and enoyl-ACP reductase. As shown on the right of the
diagram, the products of FAS depend on the contributions of various KASes, the substrate and
double bond specificities of acyl-ACP desaturases, and the substrate specificities of thioesterases
that release fatty acids for export from the plastids. In the ER, phosphatidic acid (PA) is assembled
by sequential activities of glycerol-3-phosphate acyltransferase (G3P-AT) and lysophosphatidic
acid-acyltransferase (LPAAT). Diacylglycerol (DAG) units released from lyso-PA by phosphatidate
phosphatase may be converted directly to triacylglycerol by DGAT. However, a large proportion |





