Fatty acid composition of triacylglycerols
Lipids are also main components of the human diet. The consumer preference for plant-derivedoils is increasing to the detriment of animal fats. Annual plant oil production is increasing worldwide and most of it is used for human consumptionas margarines, oils and food ingredients. The triacylglycerolsare the most important components of plant seed oils. Interestingly, the physical and chemical properties of an edible oil are related to the chemical structure of the fatty acidsesterifying the glycerol (Table6.1). Properties such as melting point, colour, flavour, mouthfeel, spreadability, stability, and effects on human health are determined by the fatty acid composition of the triacylglycerols. Most efforts in developing changes in the lipid composition of plant oils have been directed
to change the proportion among the fatty acids of the triacylglycerols.
Common fatty acids in the commercial seed oils are lauric, myristic, palmitic,
stearic, oleic, linoleic and linolenic. As is apparent, their differences occur in the
length of the carbon skeleton (C12 to C20) as well as in the presence of double
bonds (unsaturations). Long chain fatty acids containing two or more double bonds are named polyunsaturated fatty acids (PUFA). Different studies on the
effect of dietary fatty acids consumption on human health have noticed the trend
of consumers towards a reduction of saturated acids in the diet and, accordingly,
an increase in unsaturated acids. Epidemiological studies have shown that intake
of monounsaturated acids was associated with a low incidence of coronary
artery disease (Keys
et al., 1986), which has been explained by its reduction in
the low density lipoproteins (LDL) levels and their oxidation (Mata
et al., 1997).
| |
Table 6.1 Nomenclature and represntative examples of naturally occuring fatty acids |
|
|
|
| |
Systematic nomenclature |
m : n ΔaZbZ... |
|
|
|
| |
|
|
m : carbon atoms |
|
|
| |
|
|
n : double bonds |
|
|
| |
|
|
superscript : position of the double bonds |
|
| |
|
|
a, b, ... : carbon numbered from the carboxyl end |
|
| |
|
|
Z : configurationcis of the double bond |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
| |
Examples: |
|
|
|
|
| |
Saturated |
18 : 0 |
Stearic acid |
|
|
| |
Unsaturated |
18 : 1Δ9Z |
Oleic acid |
|
|
| |
Polyunsaturated (PUFA) |
20 : 5Δ5Z,8Z,11Z,14Z,17Z |
Eicosapentaenoic acid |
|
|
|
|
|
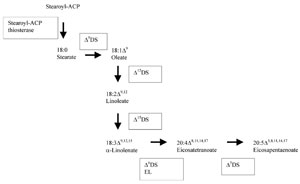
Fig. 6.2 Interconversionsof the fatty acidsindicatedin Table6.1 asexamples, Enzymes: ΔnDS: n-desaturase: EL: elongase.
Therefore the unsaturation of fatty acids have been the target for modification by
genetic engineering studies. Although the metabolic pathways leading to the
synthesis of these compounds are not simple, some genes have been adequately
selected to be modified. Oleic acid, the major monounsaturated acid of the diet,
reduces cholesterol and LDL in the serum. Transgenic plants over expressing the
desaturase gene, that encodes for the enzymecataly sing the conversion of the
saturated precursor stearic acid C18 into oleic, have been obtained (Fig. 6.2).
This has determined that oleic content of soybean has been raised to values upto
80% of the total fatty acids content of the seeds (Kinney, 1996). Although the
relationship between PUFA and disease remains contentious, there is a
consensus among the health organisations that PUFA should form 8–23% of
the total lipid intake in the human diet (Gill and Valivety, 1997).
However, the
production of high PUFA oil plants is not straight forward (Fig. 6.2), and no
report on transgenic plants with high PUFA contentis known.

Fig. 6.2 Interconversionsof the fatty acidsindicatedin Table6.1 asexamples, Enzymes: ΔnDS: n-desaturase: EL: elongase.
Interestingly, there have been cases where saturation of the fatty acids has
been the purpose of the plant genetic modification. Saturation of fatty acids
determines properties such as melting temperature and viscosity that may be
important to a commercial product. Margarine, for example, needsto be easily spreadable with in a range of temperatures. In addition, saturation may also be
beneficial for oil stability since it is known that unsaturated acids are more
readily oxidised, resulting in an increased tendency to rancidity and off odours.
Finally, vegetable oils used for frying require partial saturation by
hydrogenation in order to give adequate characteristics of stability and melting
temperature to these oils. The chemical hydrogenation has been proven to
induce also a change in the configuration of the double bonds of the fatty acids,
from the naturally occurring
cis to
trans. The presence of the
trans unsaturated
fatty acids has been correlated to a risk of coronary heart disease. Therefore,
plants with a high content of natural oil and with a high level of saturation have
been engineered. High stearate content of the oil in a
Brassica plant has been
achieved by two methods. One method transformed this plant species with the
antisense construct of the gene encoding the stearoyl-ACP desaturase, the
enzyme that catalyses the transformation of stearic acid into oleic acid (Fig. 6.2,
Δ
9DS) (Knutzon
et al. 1992). The silencing of the endogenous gene produced
the accumulation of stearic acid up to 40% of the total fatty acids content. The
second method transformed the
Brassica plant with a gene encoding the stearoyl
-ACP thiosterase specific for the synthesis of stearic acid (Fig. 6.2). Using this
approach, the transgenic plants yielded up to 68% of this fatty acid.






