Paraxial (Paraxonemal) Rod
In addition to the 9+2 axoneme, algal flagella may contain a number of other structures within the
flagellar membrane. Among those extending through the entire length of the flagella, there is the paraflagellar rod (PFR), which is known only in the order of Pedinellales (Chrysophyceae, Heterokontophyta), and in members of the Euglenophyta and Dinophyta. PFRs are complex and highly
organized lattice-like structures that run parallel to the axoneme. Among the different groups,
PFRs are very similar structurally and biochemically.
In the Pedinellales, the membrane of the single emergent flagellum is expanded into a sheath or
fin supported along the edge by the rod, which is cross-banded. Owing to the presence of the fins,
these algae are excellent swimmers. Their axoneme beats in a distinct planar wave. Paraflagellar
structures of characteristic appearance are present in some dinoflagellates.
A hollow cylinder with the wall composed of helically arranged filaments is present in the single emergent (longitudinal) flagellum of Noctiluca gametes, and in the longitudinal flagellum of both Gyrodinium lebouriae and Oxyrrhis marina. This rod is about as big as the axoneme in diameter and runs along the axoneme to which it is attached for almost its entire length. The thin filaments (nanofilaments with a 2–4 nm diameter) of its highly geometrically organized network are periodically attached to some of the outer doublet microtubules of the axoneme, which in O. marina is doublet no. 4. A different type of paraxial rod is present in the complex-shaped transverse flagellum of dinoflagellates. This rod takes a nearly straight path along the inner wall of the sulcus and is regularly banded in the transverse direction. In contrast, the axoneme itself is distinctly helical.
A rod is present in most members of Euglenophyta. In genera with two emergent flagella, such
as Eutreptia and Eutreptiella, both flagella carry a paraxial rod. In Eutreptiella, where the two
flagella differ in length, the rods differ from each other in thickness and fine structure, the longer
flagellum carrying a more complex rod than the shorter flagellum. In genera with a flagellar apparatus
reduced to one long emergent flagellum and one short flagellum not extending beyond the reservoir region, only the emergent flagellum retains the rod, which extends its entire length. An example is Euglena gracilis; in this alga, the rod arises just above the flagellar transition zone and is located latero-ventrally with respect to the axoneme and the cell body. In cross-thin sections of isolated and demembranated flagella, the rod appears hollow with an outer diameter of 90 nm. Images obtained from negative staining preparations show that the rod is made up of several coiled filaments, with a diameter of 22 nm, forming a seven-start left-handed helix with a pitch of 458 and a periodicity of 54 nm. Extending from the surface of the rod a series of goblet-like projections can be observed, which form the point of attachment between the rod and one of the axonemal doublet microtubules (Figure 2.37a and 2.37b). The PFR does not assume any consistent orientation with respect to the central-pair microtubules of the emergent flagellum.
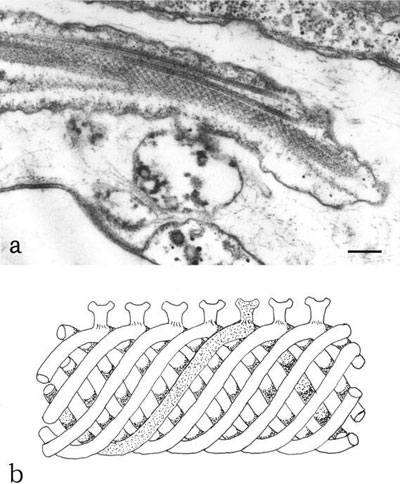
FIGURE 2.37 Transmission electron microscopy image of the locomotory flagellum of E. gracilis in longitudinal section showing the PFR (a). Schematic drawing of the PFR showing the coiled filaments and goblet-like projections (b). (Bar: 0.40 µm.)
Two major protein components of the PFR have been identified in euglenoids, and dinoflagellates
with a number of possible minor protein constituents. These major proteins (referred from now
as PFR1 and PFR2) migrate in the SDS–PAGE as a doublet of similar abundance. Depending on the
organism, the mobility for PFR1 ranges from 70 to 80 kDa, and for PFR2 from 62 to 70 kDa. Coiledcoils
are a common structural motif in the filament formed using the PFR1 and PFR2 proteins. Database searches reveal a 41-residue conserved region of the PFR1/PFR2 family that bears a significant relationship to a conserved motif within the central coiled-coil rod of tropomyosin.





