Determination of Km and Vmax
- Enzyme extract
- 8 mM L-DOPA, pH 6.6
- Spectrophotometer and cuvettes
- Stopwatch
Procedure
- Dilute the DOPA standard (8 mM) to obtain each of the following concentrations of L-DOPA: 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM.
- Repeat Exercise 7 for each of the substrate concentrations listed, substituting the change in concentration where appropriate.
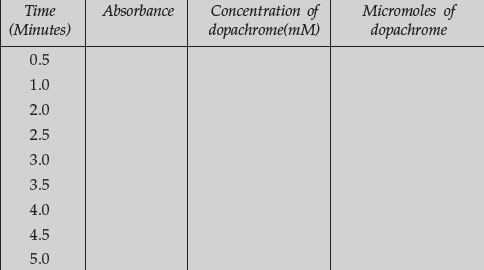
- Plot each set of data and from the data calculate the time required to convert 10 micromoles of DOPA to dopachrome. Compute the velocity of enzyme reaction for each substrate concentration. Fill in the following table:
- Plot the rate of DOPA conversion (v) against substrate concentration. This is a Michaelis-Menten plot.
- Plot a double reciprocal of the values plotted in step 4; that is, 1/s versus 1/v. This is a Lineweaver-Burk plot.
- Perform a linear regression analysis on the second plot and compute the slope and both y- and x-intercepts. Note that the x-intercept is –1/Km, the negative inverse of which is the Michaelis-Menten Constant. The y-intercept is 1/Vmax and the slope equals Km/Vmax




