Isolation of Restriction Fragments from Agarose Gels by Collection onto DEAE Cellulose
A DNA restriction fragment is isolated by collection on DEAE cellulose paper during electrophoresis, then washed from the DEAE with a high salt buffer, cleaned, precipitated, and resuspended in a small volume. Recovery of 50%–90% of the bound DNA can be expected; however, fragments larger than 7 kb have lower yields. DNA prepared this way is suitable for subcloning.
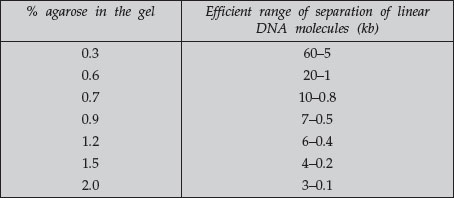
A DNA fragment of a given size migrates at different rates through gels containing different concentrations of agarose. By using a gel at the appropriate gel concentration, it is possible to resolve well the DNA of interest. Use the following table as a guide for determining the agarose concentration to use.
 |
|---|
Time Required
- 3–4 hours on Day 1
- 2–4 hours on Day 2
- Schleicher & Schuell NA-45 DEAE membrane.
Day 1
Isolating the fragment:
- Run the restriction digest and the appropriate size markers on a 1X Tris- Borate agarose gel with ethidium bromide. Be sure to leave at least 1–2 wells between samples. Run the gel until the DNA bands are well separated (visualize on the long-wavelength UV lightbox).
- Cut a slit just ahead of the band of interest using a sharp sterile razor blade or scalpel. Using blunt-edged forceps (such as Millipore forceps), carefully insert an NA-45 paper into the slit (prewet and cut NA-45 to the width of the band, see preparation of the NA-45 below).
- Place the gel in fresh 1X Tris-Borate buffer, and run the gel until the fragment has moved out of the gel and stopped by the NA-45. Monitor the progress of the band with the handheld long-wavelength UV light. Do not allow other bands of higher molecular weight to run onto the NA-45.
- Remove the NA-45 paper, rinse in NET buffer, and place in a labeled eppendorf tube. Add sufficient high-salt NET buffer to cover most of the membrane (typically 150–300 µL). Spin 5 seconds in a microcentrifuge to submerge the entire strip. Place at 65°C for 1 hour, mixing frequently, and respinning if the membrane rides up the side of the tube.
- Transfer the buffer (+DNA fragment) to a clean, labeled tube. Wash the membrane (in the original tube) with 50 mL high-salt NET buffer and add the wash to the DNA fragment tube.
- To remove ethidium bromide, extract twice with 3 volumes water-saturated n-butanol.
- Precipitate the DNA with 2.5 volumes of ethanol at –20°C for at least 1 hour (can sit overnight in the freezer).
- Pellet the DNA (for 20 minutes at high speed in a microcentrifuge) and resuspend in 50 µL TE. Reprecipitate with sodium acetate to remove any residual NaCl. Add 5 mL 3M Na-acetate, and 120 µL ethanol, hold at –20°C for 2 hours or more, pellet as before, and resuspend in an appropriate amount of TE.
Solutions
- Preparation of the DEAE cellulose membrane. Schleicher and Schuell NA-45 can be used as supplied by the manufacturer, with prewetting in sterile dH2O. However, the binding capacity of the membrane is increased with the following: a 10-minute soak in 10 mM EDTA pH 7.6, then 5 minutes in 0.5 N NaOH, followed by several rapid washes in sterile dH2O. Membranes can be stored for several weeks in sterile dH2O at 4°C.
- NET buffer (500 mL). 25 mL 3 M NaCl, 150 mM NaCl, 100 mL 0.5 M EDTA, 100 mM EDTA,10 mL 1 M Tris pH 7.5, 20 mM Tris pH 7.5, 365 mL dH2O. Autoclave to sterilize.
- High salt NET buffer (500 mL). 166.7 mL 3M NaCl, 1 M NaCl, 100 mL 0.5 M EDTA, 100 mM EDTA,10 mL 1M Tris pH 7.5, 20 mM Tris pH7.5, 223.3 mL dH2O. Autoclave to sterilize.




