Staining Chromosomes (G-Banding)
To stain metaphase chromosomes with Giemsa or Leishman’s stain to elicit a banding pattern throughout the chromosome arms, designated G-Bands. This G-Banding technique requires a chromosomal pretreatment step of trypsin to induce chromosome bands.
Time Required
30 to 60 minutes
Special Reagents
- Leishman stain
- Gurr buffer tablets
Procedure
- Prepare the staining solution the day prior to use. Also, slides should be aged at least 7–10 days or placed in a 55°C–65°C oven for 45 minutes before staining, to ensure excellent banding patterns. Aging the slides helps to eliminate fuzzy banding and increases contrast of the bands.
- Exact timing is important; therefore, a maximum of 5 slides should be stained at one time. Optimum time in the stain appears to be between 2.5–4 minutes. It is necessary to determine the approximate staining times for each bottle of stain solution. The exact time will vary by several seconds depending on the source of cells, age of slides, the cell concentration on the slide, etc. (refer to the table below)
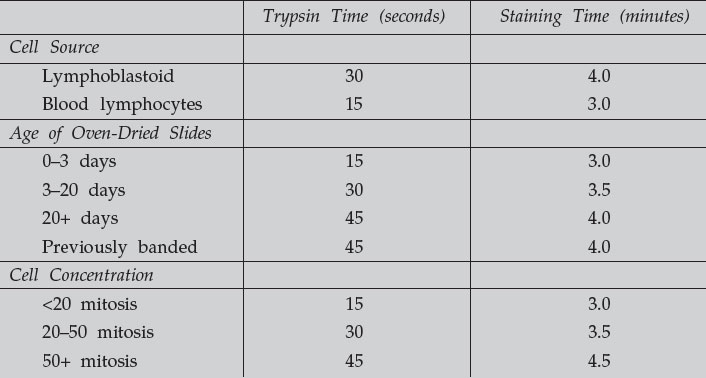
These are approximate times, and test slides need to be done to determine trypsin
and staining time for each cell line.
- Mix 1 mL 0.25% stock trypsin with 49 mL 0.85% NaCl (working salt solution). Wait 4 minutes before beginning to stain to allow the trypsin to dissolve.
- Dip oven-dried slides that have cooled to room temperature in the trypsin solution for 5–30 seconds. The time in trypsin is dependent on slide preparation conditions, harvesting conditions, material being banded, etc. Stain a test slide first to determine optimum conditions.
- Rinse slides in 50-mL working salt solution.
- Use a graduated cylinder to mix 15 mL Leishman stain and 45 mL Gurr buffer just prior to staining, and pour into a coplin staining jar. Stain the slides for 3 to 4 minutes.
- Rinse slides in distilled water and air dry with compressed air or use a blow dryer.
- Check the slides using a Zeiss Microscope, 100X plan-apochromatic oil objective, brightfield. See “brightfield photography”.
- Overtrypsinized chromosomes appear fuzzy; somewhat difficult to recognize exact bands.
- Undertrypsinized chromosomes will have indistinct bands, decreased contrast; very difficult or impossible to determine bands.
- Adequately trypsinized chromosomes will show telomeres not overly digested and G-Bands will appear sharp and in contrast.
- If slides are undertreated with trypsin, destain the slides before rebanding. Quickly dip the slides in 3:1 ethanol:acetic acid 2 or 3 times, or until all stain is removed. Rinse in distilled water, air dry, and reband.
- Coverslip with 24 × 50 mm #1 coverslip using permount.
- Transfer slides to a microslide box or suitable storage container.
Solutions
- Trypsin (1:250) USP Grade; porcine parvovirus tested. Prepare a 0.25% (1X) solution by dissolving 0.25 g trypsin in 100 mL PBS. Aliquot into 1 mL quantities and store at –20°C for up to 1 year. Thaw just before using. Lowering thetemperature of the trypsin in the working salt solution will lower its activity, thus requiring longer exposure times and also increasing the life of the solution. Test slides should be run to determine optimum exposure times.
- Gurr Buffer Solution: (1 liter). 1 tablet is dissolved in 1 liter distilled water. Produces a solution of pH approximately 6.8 at 20°C. Use solution at room temperature.
- Working Salt Solution: (1 liter). Dissolve 8.5 g of NaCl in 1 liter distilled water. Use at room temperature.
- Leishman Stain Solution. Dissolve 1 g Leishman stain into a 500-mL bottle of methanol. Stir for 4 hours and allow stain to age at least 1 day prior to use. If stain is not dissolved, filter stain through a 0.45-µm filter.




