Determination of Antibody Specificity by Western Blotting
Recent advances in functional genomics, particularly in gene and protein expression profiling technologies (Figeys 2002; Panisko et al., 2002; Gerling and Solomon, 2003; Valle and Jendoubi, 2003), have underlined the importance of antibodies to rapidly validate the results in both cultured cells and tissues (see other articles in this volume). The utility of antibodies, however, depends very much on their specificity, and today immunoblotting (Towbin et al., 1979; Symington, 1984; Harlow and Lane, 1988; Otto and Lee, 1993), in combination with high-resolution two-dimensional electrophoresis (see articles by Celis et al. and by G6rg and Weiss), provides the most efficient procedure to determine their specificity.
II. MATERIALS AND INSTRUMENTATION
Tris base (Cat. No. 648311) and Nonident P-40 (NP- 40, Cat. No. 492015) are from Calbiochem. Glycine (Cat. No. 808822) is from ICN Biomedicals. Thirty percent hydrogen peroxide (Cat. No. 7209), dehydrated skim milk (Cat. No. 1.15363), and Tween 20 are from Merck. Peroxidase-conjugated antimouse immunoglobulins (Cat. No. P0447) and peroxidaseconjugated antirabbit immunoglobulins (Cat. No. P0217) are from DAKO. The monoclonal antibodies against MEK2, crk-1, NCK, and p27 are from Transduction Laboratories. The polyclonal antibodies against hnRNP K and stratifin are from our laboratory. The monoclonal antibody against hsp28 was kindly provided by A.-P. Arrigo. The HRP colour development reagent (Cat. No. 170-6534) is from Bio-Rad. Nitrocellulose Hybond-C (Cat. No. RPN 203C), the ECL kit (Cat. No. RPN 2106), Lumigen TMA-6 or ECL Advance Western blotting detection kit (Cat. No. RPN 2135), and ECL Advance Western blocking kit are from Amersham. Tissue culture media and supplements are as described elsewhere (Celis and Celis, 1998; see also article by Gromov and Celis). Plates (96 wells, Cat. No. 655180) are from Greiner. Rectangular (24 × 19cm) dishes are from Corning (Cat. No. PX 38567), and Xray films (X-Omat UV; 18 × 24cm, Cat. No. 524 9792) are from Kodak. The Trans-Blot electrophoretic transfer cell is from Bio-Rad (Cat. No. 170-3910), the orbital shaker (Red Rotor PR75) is from Pharmacia, and the roller bottle-type blotter (Navigator, Cat. No. 128-10T1) is from Biocomp.
The procedure is illustrated using whole protein extracts obtained from noncultured human keratinocytes and the bladder TCC cell line RT4. Proteins are separated by two-dimensional (2D) gel electrophoresis (see article by Celis et al.) blotted to a nitrocellulose membrane (Towbin et al., 1979), and developed using the HRP colour development reagent and/or the ECL procedure.
A. Blotting
Solution
Tris, glycine, methanol (TGM): 25mm Tris, 192mM glycine, and 20% (v/v) methanol. To make 5 liters, weigh 15.14g of Tris base and 72.07 g of glycine. Add 1 liter of methanol and complete to 5 liters with distilled water.
Steps
- Prepare the protein extracts and run 2D gels as described in the article by Celis et al. Mix the unlabeled cell extract in O'Farrel lysis solution with a small amount of [35S]methionine-labeled proteins from the same source (about 500,000cpm; for labelling of cells; see article by Celis et al.) in order to facilitate the immunodetection of the antigen(s). For 1D blots, resuspend the samples in Laemmli's buffer (Laemmli, 1970).
- Place a piece of 3MM Whatmann paper, a bit larger than the size of the gel, in a rectangular glass pie dish (24 × 19 cm) containing about 100 ml of TGM. Place the gel on top of the paper.
- Equilibrate for 5 min at room temperature.
- Wet the fiber gel pads in TGM.
- Open the gel holder (Fig. 1C) and place one fiber gel pad in each side.
- Wet the nitrocellulose membrane (14 × 16cm) in TGM by capillary action as shown in Fig. 1A. Use gloves to handle the membranes.
- Place the wet nitrocellulose membrane on top of the gel (Fig. 1B). The operation should be done under the buffer. Rub the membrane from one end to the other to eliminate bubbles.
- Place another wet 3MM Whatmann paper on top of the nitrocellulose membrane. Rub the paper carefully to avoid bubbles. Lift the "sandwich" (same side up) and place it on top of the fiber gel pad located on the black holder (Fig. 1C).
- Close the gel holder and place it in the Trans-Blot tank containing about 750ml of TBM. The black side of the holder should face the cathode side (indicated with black in the tank). Up to three holders can be inserted in the tank. If only one cassette is used, insert in the middle track.
- Fill the tank with TGM, connect the electrodes, and run at room temperature for 24h at 130mA.
- Following protein transfer, dry the membranes and expose to an X-ray film to assess the quality of the transfer (Fig. 3A). Dry sheets can be kept for extended periods of time without significant changes in the reactivity of the proteins. Blots that do not contain radioactivity can be stained with amido black.
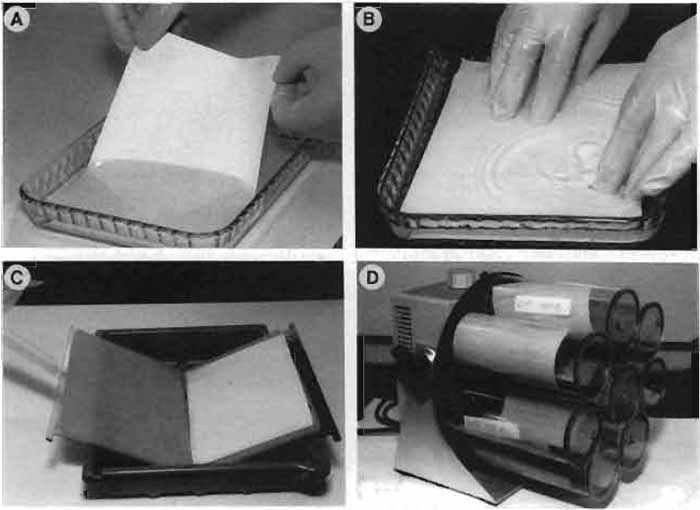 |
| FIGURE 1 (A) Wetting the nitrocellulose membrane. (B) Placing the membrane on top of the gel. (C) Holder with fiber gel pads. (D) Rotating roller system (Navigator). |
B. Immunodetection
1. HRP Colour Development
- Hank's buffered saline solution (HBSS) without Ca2+ and Mg2+: 10× stock solution. To make 1 liter, weigh 4 g of KCl, 0.6g of KH2PO4, 80g of NaCl, and 0.621 g of Na2HPO4·2H2O. Complete to 1 liter with distilled water.
- 1× HBSS without Ca2+ and Mg2+: To make 1 liter of HBSS, use 100ml of the 10× stock solution and complete to 1 liter with distilled water.
- 2× TBS stock: 0.04M Tris and 0.274M NaCl, pH 7.5. To make 1 liter, add 4.84 g of Tris base and 16 g of NaCl. Adjust to pH 7.5 with HCl and complete to 1 liter with distilled water. Store at 4°C.
- Dehydrated skim milk: 50mg/ml in HBSS
- Primary antibody: Primary antibodies should be diluted to the a volume of 8 ml. The dilution (1:10 to 1:10,000) should be optimised in preliminary experiments for the best results in terms of high signal and low background.
- Horseradish peroxidase-conju gated immunoglobulins (HRP-labeled second antibodies): HRP-conjugated secondary antibodies should be diluted to a final volume of 8ml. The dilution (1:1000 to 1:10,000) should be optimised in preliminary experiments for the best results in terms of high signal and low background
- HRP colour development solution: To make 60ml, dissolve 30mg of HRP color development reagent in 10 ml of methanol (protect from light). Mix 30ml of 2× TBS and 20ml of distilled water. Just before use add 30µl of ice-cold 30% H2O2 to the TBS and mix the two solutions.
- Cut the appropriate area of the blot with a scalpel using the X-ray film as reference and wet by capillarity in the skim milk solution. Incubate with shaking for 1 h at room temperature (or overnight in the cold room) in the same solution. Incubation can be done in a roller bottle-type blotter (Navigator Model 128, Fig. 1D) or a rectangular plastic dish (15 × 18.7cm; a minimum volume of 40ml per dish is needed). In the former case, the volume needed is considerably less (8 ml). For 1D gel strips we use the chamber shown in Fig. 2 (Pierce). The following immunodetection procedure is illustrated using the roller bottle-type blotter.
- Wash the blots three times for 10min each in HBSS.
- Add 8 ml of primary antibody diluted in HBSS. Roll for 2 h at room temperature.
- Wash three times for 10 min each with HBSS.
- Add 8ml of a 1:200 dilution of peroxidaseconjugated rabbit antimouse immunoglobulins in HBSS. Roll for 2h at room temperature.
- Transfer the blot to a rectangular plastic dish and wash three times for 10min each in HBSS.
- Prepare the HRP colour development solution just before use.
- Aspirate the HBSS and add 30ml of the HRP solution. Shake at room temperature until staining appears. Discard the HRP colour developer according to the safety regulations enforced in your laboratory.
- Rinse well with demineralised or tap water and dry. Superimpose the dry blot and the X-ray film with the help of the radioactive marks.
 |
| FIGURE 2 Chamber for incubating one-dimensional gel strips with antibodies. |
2. ECL Detection
The procedure has been slightly modified from the ECL Western blotting protocol described by Amersham.
Solutions
- 10× TBS, pH 7.6, stock: To make 2 liters, add 48.4 g Tris base (0.20 M) and 160 g NaCl (1.37 M). Adjust to pH 7.6 with HCl (37% fuming) and complete to 2 liters with distilled water. Store at 4°C.
- TBS-Tween 0.05%: To make 1 liter, add 100ml of 10× TBS, 0.5 ml of Tween 20, and complete to 1 liter with distilled water.
- Blocking solution: 5% skim milk; 5g per 100ml of TBS-Tween.
- Primary antibody: Primary antibodies should be diluted to a final volume of 8 ml. The dilution (1:10 to 1:10,000) should be optimised in preliminary experiments to achieve high signal and low background.
- Horseradish peroxidase-conju gated immunoglobulins (HRP-labeled second antibodies): HRP-conjugated secondary antibodies should be diluted to a final volume of 8ml. The dilution (1:1000 to 1:10,000) should be optimised in preliminary experiments to achieve high signal and low background.
- Wet the nitrocellulose blot with TBS-Tween in a rectangular plastic dish and transfer it to a bottle (fitting the Nagivator system, Fig. 1D) containing 8 ml of blocking solution.
- Block for 1 h at room temperature or overnight in a cold room.
- Rinse the membranes briefly two times in TBS-Tween and wash three times for 10min each.
- Add 8ml of the primary antibody diluted in TBS-Tween and incubate for 1.5h at room temperature.
- Rinse the membranes briefly two times in TBS-Tween and wash three times for 10min each.
- Add 8ml of HRP-labelled secondary antibody diluted in TBS-Tween for 1 h at room temperature.
- Transfer the membrane blot to a plastic dish, rinse briefly two times in TBS-Tween, and wash three times for 10 min each. The next steps are carried out in a dark room.
- Prepare the detection reagent by mixing 1 ml of detection solution 1 with 1 ml of detection solution 2. This amount is sufficient for one 13 × 15-cm blot.
- Lift the blot carefully and dry it gently by touching a piece of paper towel. Place the blot on a piece of Saran wrap or on another transparent plastic sheet, protein side up. Add the detection reagent to the protein side of the blot, cover it quickly with another piece of Saran wrap, and smooth out air pockets gently.
- Incubate for I min at room temperature.
- Drain off excess detection reagent with a soft kitchen paper and place the sandwich in the film cassette. Try to work as quickly as possible. The next step is carried out in a dark room.
- Turn off the light (red safety light is allowed). Place an X-ray film on top of the membrane and close the cassette. Expose for 5, 15, and 30 s and up to 15 min if necessary.
Figure 3 shows several IEF 2D gel blots of protein from noncultured human keratinocytes reacted with antibodies against crk-1, galectin 1, hnRNP K, NCK, hsp28, and p27 antibodies (Figs. 3B). The positions of the corresponding spots in the autoradiograms are indicated (Fig. 3A).
![FIGURE 3 Two-dimensional ECL immunoblots of proteins from a bladder transitional cell carcinoma labelled with [35S]methionine (A) and reacted with antibody (B) against crk-1 (a), galectin 1 (b), hnRNP K (c), NCK (d), hsp28 (e), and p27 (f).](images/v1_pC_s15_c63_f03.jpg) |
| FIGURE 3 Two-dimensional ECL immunoblots of proteins from a bladder transitional cell carcinoma labelled with [35S]methionine (A) and reacted with antibody (B) against crk-1 (a), galectin 1 (b), hnRNP K (c), NCK (d), hsp28 (e), and p27 (f). |
While the ECL chemiluminescent detection system for assaying proteins yields rapid luminescence with a good signal-to-noise ratio, such luminescence is short lived and of modest intensity. This detection method is thus significantly limited in its ability to detect small amounts of the target analyte. Therefore, assays of longer duration and/or higher sensitivity are required in cases of limiting amounts of sample/antibody or proportionally minute amounts of target analyte in a sample. Several high-sensitivity substrates, such as the SuperSignal West substrates (Pierce Biotechnology, Inc.), Lumigen PS-3, and TMA-6 (Lumigen, Inc.), have been developed to address this need. The following protocol is based on an ultrasensitive HRP substrate, Lumigen TMA-6 (commercialised by Amersham as ECL Advance western blotting detection kit), as an alternative to the standard ECL detection method. This procedure is particularly useful for low abundance proteins or limited availability of antibody.
Solutions
- 10× TBS, pH 7.6, stock: To make 2 liters, add 48.4 g Tris base (0.20M) and 160g NaCl (1.37M). Adjust to pH 7.6 with HCl (37% fuming) and complete to 2 liters with distilled water. Store at 4°C.
- TBS-Tween 0.2%: To make 1 liter, add 100ml of 10× TBS, 2 ml of Tween 20, and complete to 1 liter with distilled water.
- Blocking solution: 2% ECL Advance blocking agent; 2 g per 100 ml of TBS-Tween
- Rabbit polyclonal antistratifin (14-3-3σ) peptide antibody
- Wet the nitrocellulose blot with TBS-Tween in a rectangular plastic dish and transfer it to a bottle (fitting the Nagivator system, Fig. 1D) containing 10 ml of blocking solution.
- Block for 1 h at room temperature or overnight in a cold room.
- Wash twice for 1 min in TBS-Tween.
- Add 10ml of the primary antibody, in this case rabbit polyclonal antistratifin (14-3-3o) diluted 1 : 15,000 in blocking solution, and incubate for 1 h at room temperature.
- Wash three times for 5 min and once for 15 min in TBS-Tween.
- Briefly rinse the membrane with two changes of TBS-Tween.
- Add 10 ml of peroxidase-conjugated secondary antibody diluted 1:10,000 in blocking solution for 1 h at room temperature.
- Transfer the membrane blot to a plastic dish and wash three times for 5 and once for 15min in TBS-Tween.
- Briefly rinse the membrane with two changes of TBS-Tween.
- Mix 5 ml of detection solution A with 5 ml of detection solution B. This is sufficient for at least one 13 × 15cm blot.
- Lift the blot carefully with forceps and dry it gently by touching the edge against a piece of paper towel. Place the blot in a clean plastic dish and add the detection solution. Leave for 5 min. Do not shake.
- Let the solution run off the blot as described earlier and put it on top of a piece of plastic in a film cassette (protein side up). Cover it quickly with Saran wrap and carefully smooth out air pockets with a piece of paper. The next steps are carried out in a dark room.
- Turn off the light (red safety light is allowed). Place an X-ray film on top of the membrane and close the cassette. Expose for 5, 15, and 30s and up to 1 h if necessary.
Figure 4 shows an IEF 2D gel blot of [35S]methioninelabeled proteins from RT4 cells reacted with the antistratifin (14-3-3o) peptide antibody.
![FIGURE 4 Two-dimensional immunoblot of proteins from the bladder cancer cell line RT4 labelled with [35]Smethionine (A) reacted with a peptide antibody against 14-3-3o (B) and developed with Lumigen TMA-6.](images/v1_pC_s15_c63_f04.jpg) |
| FIGURE 4 Two-dimensional immunoblot of proteins from the bladder cancer cell line RT4 labelled with [35]Smethionine (A) reacted with a peptide antibody against 14-3-3o (B) and developed with Lumigen TMA-6. |
A. Blotting
- Remove excess vaseline floating in the buffer with a tissue.
- Avoid air bubbles when making the "sandwich" and lifting it to the fiber gel pad.
- For 2D gel blotting, it is important to run a range of concentrations of the protein mixture. Choose a protein concentration that does not give streaking. Never run more protein than is necessary.
- Membrane proteins may streak and it may be necessary to use special detergents (Santoni et al., 2000; Tastet et al., 2003)
B. HRP Colouring Development
H2O2 is unstable. Check the expiration date on the bottle.
C. ECL Detection
- It is essential to determine optimal antibody concentrations using dot blots. Be aware that some antibodies may require dilutions of up to 1:100,000 when using the ECL Advance Western blotting kit.
- Use abundant volumes of buffer during the washing steps.
- It is important to use an optimised the amount of blocking agent. We usually use the ECL Advance blocking agent, but other blocking agents (e.g., SuperBlock, Pierce Biotechnology Inc.) can be used and, in certain circumstances, perform better. As signal emission is stable for several hours, maximal reduction of background noise and nonspecific binding allows detection of even minute amounts of the target protein.
References
Celis, J. E., et al. (1991). The master two-dimensional gel database of human AMA cells proteins: Towards linking protein and genome sequence and mapping information. Electrophoresis 12, 765-801.
Figeys, D. (2002). Functional proteomics: Mapping protein-protein interactions and pathways. Curr. Opin. Mol. Ther. 4, 210-215.
Gerling, I. C., Solomon, S. S., and Bryer-Ash, M. (2003). Genomes, transcriptomes, and proteomes: Molecular medicine and its impact on medical practice. Arch. Intern. Med. 163, 190-198.
Harlow, E., and Lane, D. (1988). "Antibodies: A Laboratory Manual." Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227, 680-685.
O'Farrell, P. H. (1975). High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250, 4007-4021.
O'Farrell, P. Z., Goodman, H. M., and O'Farrell, P. H. (1977). High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12, 1133-1142.
Otto, J. J. (1993). Immunoblotting. In "Antibodies in Cell Biology" (D. J. Asai, ed.), pp 105-117. Academic Press, San Diego.
Santoni, V., Molloy, M., and Rabilloud T. (2000). Membrane proteins and proteomics: un amour impossible? Electrophoresis 21, 1054-1070.
Symington, J. (1984). In "Two-Dimensional Gel Electrophoresis of Proteins: Methods and Applications" (J. E. Celis, R. Bravo, eds.), pp. 126-168, Academic Press, New York.
Tastet, C., Charmount, S., Chevallet, M., Luche, S., and Rubilloud, T. (2003). Structure-efficiency relationships of zwitterionic detergents a protein solubilizers in two-dimensional electrophoresis. Proteomics 3, 111-121.
Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some application. Proc. Natl. Acad. Sci. USA 76, 4350-4354.
Valle, R. P., Jendoubi, M. (2003). Antibody-based technologies for target discovery. Curr. Opin. Drug. Disco v. Dev. 6, 197-203.




