Rapid Development of Monoclonal Antibodies Using Repetitive Immunizations, Multiple Sites
An in-depth understanding of both the formation of germinal centers and the immunoregulatory processes involved in T-cell-dependent B-cell responses (Levy et al., 1989; Berek et al., 1991; Jacob et al., 1991; Kroese et al., 1990; Nossal, 1992; MacLennan, 1994; Kelsoe, 1996) led us to initially explore the feasibility of modifying immunization and fusion time lines used for developing monoclonal antibodies. Our studies demonstrated that monoclonal antibodies could be generated quickly using an immunization and somatic fusion strategy, which we refer to as repetitive immunizations, multiple sites (RIMMS) (Kilpatrick et al., 1997). Immunizations, somatic fusion, screening, and isolation of affinity-matured IgG-secreting hybridoma cell lines can be achieved within I month. RIMMS capitalizes on somatic fusion of immune B cells undergoing germinal center maturation in draining lymph nodes (Kilpatrick et al., 1997, 2003). The immunization sites used for RIMMS are proximal to easily accessible regional lymph nodes. RIMMS involves the use of P3X63/Ag8.653 murine myeloma cells stably transfected with human BCL-2 (Kilpatrick et al., 1997). Fusions can be performed as early as 7 days (Bynum et al., 1999) out to 14 days after the onset of immunization using recombinant protein, conjugated synthetic peptides, or drug haptens (Kilpatrick et al., 1997; Ignar et al., 1998; Kinch et al., 1998; Wring et al., 1999; Alligood et al., 2000; Ellis et al., 2000; Lindley et al., 2000). RIMMS has also been used successfully to generate high-affinity antibodies using DNA-based immunizations in conjunction with the PowderJect gene gun (Kilpatrick et al., 1997, 1998, 2000, 2002, 2003; Kinch et al., 2002).
II. MATERIALS AND INSTRUMENTATION
Fine curved forceps (Cat. No. 1-23-20) and microdissecting scissors (Cat No. 11-250) are from Biomedical Research Instruments. The following items are from Corning Costar: 0.2-µm vacuum filter/storage units (Cat. No. 431205), T 25-cm2 tissue culture flasks (Cat. No. 3056), and 96-well tissue culture plates (Cat. No. 3595), as well as 24-well tissue culture plates (Cat. No. 3524). Items purchased from Sigma include Freund's complete adjuvant (FCA) (Cat. No. F-5881), Hybri- MAX azaserine hypoxanthine, 50× (Cat. No. A9666), Hybri-MAX dimethyl sulphoxide (DMSO) (Cat. No. D2650), deoxycholic acid sodium salt (Cat. No. D- 6750), and Igepal CA 630 (Cat. No. 1-3021, used in place of NP-40). The following products are from InVitrogen: 0.45-µm nitrocellulose (Cat. No. LC2001), RPMI 640 (Cat. No. 11875-119), 10mM, 100× nonessential amino acids (Cat. No. 11140-050), L-glutamine, 29.2mg/ml with 10,000 units penicillin, 10,000 µg/ml streptomycin (Cat. No. 10378-016), gentamycin reagent, 50mg/ml (Cat. No. 10131-035), 8-16% Tris-glycine gels (Cat. No. EC6045), and SeeBlue Plus2 markers (Cat. No. LC5925), as well as pcDNA3.1+ (Cat. No. V790-20), Topo TA cloning (Cat. No. K4500-01), imMedia Amp (Cat. No. Q600-20), imMedia Amp Agar (Cat. No. Q601-20), and ethidium bromide (Cat. No. 15582-018). NUNC 1.8-ml freezer vials (Cat. No. 66021-986), as well as 10x phosphate-buffered saline (PBS) (Cat. No. EX-6506), are from VWR. Defined fetal bovine serum (FBS) (Cat. No. SH30070.03) is from Hyclone, and Origen cloning factor (Cat. No. 210001) is from Igen. JRH EX-Cell 610 HSF medium (Cat. No. 14610- 1000M) is from JRH Bioscience. The P3-X63Ag8.653 cells (ATCC CRL-1580) and PEG 1450, MW 1300-1600, 2-g bottle are from ATCC. The RIBI adjuvant (Cat. No. R-700) is from Corixa. The anti-human BCL-2 monoclonal antibody (Cat. No. M0887) is from Dako. The following items are from Becton Dickinson: 1-ml Leur Lok syringes (Cat. No. 309626), 5-ml Leur Lok syringes (Cat. No. 309603), and 26G½ (Cat. No. 305111) and 16G1 (Cat. No. 305197) needles. BCIP/NBT color development substrate (Cat. No. S3771) is from Promega.
Eight- to 12-week-old female SJL mice are from Jackson Laboratories, and BALB/c mice are from Charles Rivers. Isoflurane (Iso Flo, Cat. No. 06-8550- 2/R1) is from Abbott Labs and is administered to mice using a Vapomatic (Model 2) from AM Bickford, Inc.
A. Preparation of Culturing Media
Solutions
- Fusion selection medium, 1 liter: Combine 500ml ExCell-610 HSF media, 260 ml RPMI 1640, 100 ml Origen hybridoma cloning factor, 100 ml FBS, 10 ml L-glutamine/ pen-strep, 10 ml nonessential amino acids, 2 vials of azaserine hypoxanthine 50×, each reconstituted with 10ml RPMI 1640. Sterile filter media in a Corning 0.2-µm vacuum filter storage unit and store at 4 °C.
- Fusion selection medium without Origen cloning factor, 1 liter: Follow the directions in solution 1, but eliminate the Origen cloning factor and increase FBS from 10 to 20%. This medium is used to eliminate background from unfused B cells following somatic fusion.
- Fusion cloning medium, 1 liter: This medium is used for limit dilution cloning of hybridomas. Combine 500ml ExCell-610 HSF media, 280ml RPMI 1640, 100 ml Origen hybridoma cloning factor, 100 ml FBS, 10 ml L-glutamine/pen-strep, and 10 ml nonessential amino acids. Sterile filter medium in a Corning 0.2-µm vacuum filter storage unit and store at 4 °C.
- Culturing medium for BCL-2-transfected P3- X63Ag8.653 (ATCC CRL-1580) cells, 1 liter: Combine 890 ml RPMI 1640, 100 ml FBS, 10 ml L-glutamine / penstrep solution, and 200µg/ml geneticin (G418). Sterile filter medium in a Corning 0.2-µm vacuum filter storage unit and store at 4 °C. One week before using the cells for fusion, pass the cells into the justdescribed medium without G418. The same medium without G418 is used to culture the parental P3- X63Ag8.653 myeloma cell line.
- Serum-free wash medium, 500ml: Add 5ml L-glutamine/ pen-strep solution to 495ml RPMI 1640, sterile filter medium in a Corning 0.2-µm vacuum filter storage unit, and store at 4 °C.
Steps
- Dose per mouse: In a 1.5-ml Eppendorf tube, add 100µl of antigen diluted in sterile PBS to a final concentration of 15µg for the primary immunization. Add 100µl of RIBI adjuvant to the tube containing the antigen and then vortex. Using a 1-ml Leur Lok syringe outfitted with a 16G1 needle, remove 100µl of Freund's complete adjuvant (vortex the vial of FCA right before use).
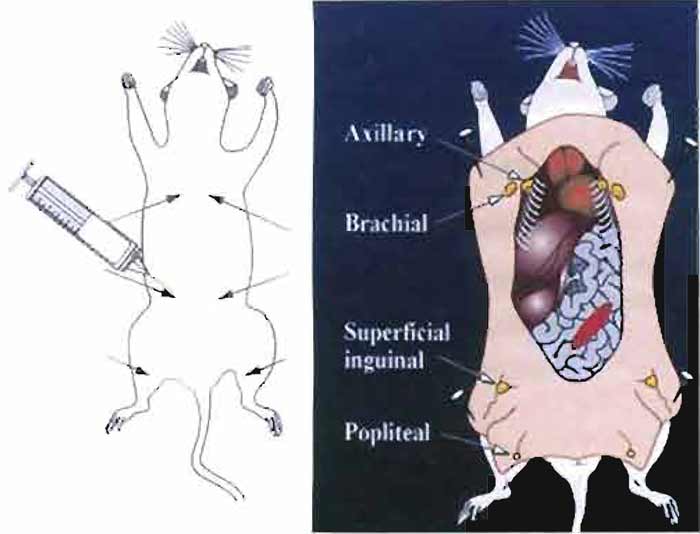
- To make an emulsion for the primary immunization, place the bevel of the needle against the inner wall at the bottom of the Eppendorf tube containing the antigen/RIBI mixture. Expel the Freund's complete adjuvant from the syringe into the tube and then draw the solution back up into the syringe holding the bevel of the needle against the inner wall of the tube. Repeat the process until a milky, slightly thickened emulsion is formed. Draw the emulsion back up into the syringe. Carefully remove the 16G needle and replace it with a 26 G ½ needle. The 300-µl volume is then delivered to the six subcutaneous sites indicated in Fig. 1, 50 µl per site, as detailed later.
- The final concentration of antigen used for the secondary immunization is 5µg, and 2-5µg of the antigen is used for the tertiary immunization. For secondary and tertiary immunizations, dilute the antigen in a 100-µl volume of sterile PBS, increase the RIBI adjuvant volume to 200 µl, and eliminate FCA.
Steps
- For each antigen, immunize two 8- to 12-weekold female SJL or two BALB/c mice (one mouse will serve as a backup) at the sites indicated in Fig. 1 on days 0, 7, and 10. Using this immunization time line, fusion can be performed on day 11, 12, or 13. Alternatively, mice can be immunized on days 0, 4, and 8, and fusions can be performed on day 11. Fusions can also be performed as early as day 7 by immunizing mice on days 0, 2, and 4 (Bynum et al., 1999). The backup mouse can be boosted every 2-3 weeks using conventional protocols (see previous article).
- Anesthetize the mice with isoflourane for all immunization time points.
- Inject 50µl of the antigen/adjuvant emulsion into six sites proximal to axillary and brachial lymph nodes (thoracic region), superficial inguinal lymph nodes (abdominal region), and popliteal lymph nodes (located behind the knee) as indicated in Fig. 1.
 |
| FIGURE 1 Immunization sites used for RIMMS are indicated by arrows. Following immunization, the axillary, brachial, superficial inguinal, and popliteal lymph nodes are exposed and then removed aseptically. Lymphocytes are isolated from the lymph nodes and are then used in a PEG-induced fusion. |
Steps
- Perform euthanasia of mice using carbon dioxide.
- Wet down the fur of the mouse with 70% ethanol.
- Lay the mouse on its back and lift up the skin in the lower groin using 70% ethanol forceps. Using 70% ethanol-rinsed microdissecting scissors, make a small incision to open up the skin (do not cut open the abdominal wall). To make a midsection incision, insert the scissors under the skin and then make an incision starting from the lower groin region up to the neck using forceps to lift up the skin. Make incisions across the top of the shoulders, down to the front feet on the left and right sides. Then make incisions from the lower groin area across to the top of the legs continuing down to the hind feet. To expose the lymph nodes, peel the skin back from the midsection incision, pull back the skin from the hind legs, and secure with pins as shown in Fig. 1.
- Using curved forceps that have been rinsed in 70% ethanol, remove the lymph nodes by placing curved microforceps under each node and then pull up gently to separate lymph nodes from surrounding tissue.
- Place the lymph nodes into a 60-mm sterile tissue culture dish containing 5ml of serum-free wash medium.
E. Isolation of Lymphocytes from Lymph Nodes
Steps
- In a laminar flow hood, remove the lymph nodes from the 60mm tissue culture dish and transfer the nodes into a new 60-mm dish containing 5ml of serum-free wash medium. Fill a 5-ml syringe with serum-free wash medium and add a 26G needle. Gently hold individual lymph nodes with 70% ethanol-rinsed curved microforceps. Insert the needle into the node and then profuse with medium in order to flush out the lymphocytes. Repeat process on each node. Using two curved microforceps, gently tease remaining cells from the capsules.
- Pipette the lymphocyte cell suspension into a 15-ml conical tube and allow the debris from the capsules of the nodes to settle to the bottom of the tube (less than 1 min). Pipette the cells away from the debris and then transfer the cell suspension into a new 15-ml conical tube. Count the cells (see article by Hoffman). For each fusion, use 3 × 107 lymphocytes (see step 3 in Section IIIF).
Note: The number of lymphocytes isolated from pooled lymph nodes will range from 1-2 × 107 (weak immunogen) to 1 × 108 when one female SJL mouse is immunized using RIMMS. You can expect one-half of this range when one female BALB/c mouse is used. - Remaining immune lymphocyte cells not used for fusion can be frozen back by pelleting the cells by centrifugation at 400g for 5 min. Resuspend the cells in 1 ml of 90% FCS, 10% DMSO freezing media. Place cells into 1 × 1.8-ml NUNC freezing vials labeled with the antigen designation and the date. Place the vial overnight at -80°C in a styrofoam container and then transfer the cells to liquid nitrogen storage the following day. These cells can be subsequently thawed and used for somatic fusion.
F. PEG-Induced Somatic Fusion
Steps
- To affect a 1:1 lymphocyte-to-myeloma ratio, harvest 3 × 107 P3-X63Ag8.653 (ATCC CRL-1580) or P3X-BCL-2-transfected myeloma cells to be used for the fusion in a 50-ml conical tube (see protocols on how to generate a bcl-2-modified fusion partner in Section IIIH). Check the viability of the myeloma cells using trypan blue; ideally you want 90-98% viability Wash the cells in serum-free wash medium by centrifuging at 400g for 5 min. Resuspend the cells in 2 ml serum-free wash medium.
- To prepare the PEG for fusion (ATCC PEG 1450, MW 1300-1600, 2-g bottle), very slowly heat the bottle on a hot plate on a low setting, just enough to melt the PEG. Do not boil the PEG. Using a 5-ml syringe outfitted with a 16G1 needle, immediately add 3ml of serum-free wash medium that has been prewarned to 37°C to make a 40% stock solution. Keep the PEG solution in a 37°C incubator until you are ready to perform the fusion. Use the PEG solution within 1.5h, as the efficiency of the fusion will drop off significantly if you use the PEG beyond 2h after preparation.
- In a 15-ml conical tube, mix 3 × 107 immune lymphocyte cells isolated from lymph nodes with 3× 107 myeloma cells (from step 1). Centrifuge the cell mixture at 400g for 5 min. Decant the media to leave a "dry pellet" by removing as much media as possible. Gently disrupt the pellet of mixed cells by tapping the bottom of the tube.
- Using a 1-ml pipette, slowly add 300µl of the PEG solution to the "dry pellet" of the myeloma and lymph node cell mixture in the bottom of the 15-cc tube. Mix gently and then allow the tube to incubate in the hood for 5 min. Gently mix the cell suspension again by tapping the tube and then allow the suspension to incubate in the hood for another 5 min.
- Using a 5-ml pipette add 4 ml of the fusion selection media to the fusion, resuspend the cells gently, and then transfer the fusion suspension into a 250-ml sterile bottle containing 196ml of fusion selection media. Swirl gently to mix cell suspension. Plate out the fusion in 10 × 96-well tissue culture plates by adding 200-µl per well.
Steps
- Avoid removing the fusion plates from the incubator for microscopic observation during the first few days after the fusion.
- Within 7 days, remove one-half of the hybridoma selection media and then replace with fresh hybridoma selection media (containing azaserine hypoxanthine). If you observe a high number of unfused lymphocytes still growing, change the media to hybridoma selection media containing 20% FBS, without Origen cloning factor. Antibody from unfused B cells will give misleading results in primary screening assays.
- Within 5-10 days you will be able to observe the outgrowth of hybridomas in the fusion plates. Harvest supernatant for ELISA analysis. ELISA positives are further tested in Western blot and immunoprecipitation.
- For limit dilution cloning for the isolation and identification of monclonal antibody-producing cell lines, please refer to the previous article.
H. Generation of a P3X-BCL-2 Myeloma Cell Line
Cloning of Human BCL-2
- 10× TBE: 108g Tris base, 55g boric acid, 40ml 0.5 M EDTA (pH 8.0)
- Ethidium bromide: 10mg/ml in dH2O
Steps
- PCR reaction mix: Mix 20pmol of primers (sense 5' cgg ggt acc gcc acc atg gcg cac gct ggg aga ac 3' and anti-sense 5' ccg tct aga tca ctt gtg gcc cag ata ggc a 3'), human BCL-2 cDNA, 10× Advantage PCR buffer, 200µM dNTP mix, dH2O, and Advantage HF polymerase.
- Set parameters for human bcl-2 in a PTC-200 Peltier thermal cycler with the following conditions. Cycle steps: a denaturation step of 3 min at 95°C to generate a hot start, 30 cycles at 95°C for 15s, 65°C for 15s, and then 72°C for l min, followed by a soak step at 4°C.
- After amplification of the cDNA, separate the PCR products and primers electrophoretically on a 1% agarose/TBE ethidium gel and purify the 642-bp band using the QiaQuick gel purification kit.
- Perform buffer exchange over a Sephadex-400 column.
- Insert the cloned cDNA into pCR2.1 using the TOPO TA cloning kit and transform into Top10 Escherichia coli.
- Prepare 6-10 cultures with 2 ml LB-amp media in a 15-ml sterile culture tube. Pick a single isolated colony with a sterile loop and inoculate each miniculture with the isolated bacteria. Incubate and shake overnight at 37°C.
- Isolate recombinants using the QiaPrep spin DNA kit and digest 1 µg of the plasmid DNA with the restriction endonucleases Asp718 and XbaI for 1 h at 37°C.
- Isolate the human BCL-2 cDNA fragment by excising the 642-bp band and then subclone into a linear pcDNA3.1(+) vector (digested with Asp718 and XbaI) using T4 DNA ligase.
- Transform 2.5 µl of the ligation reaction into chemically competent Top 10 E. coli and spread onto a LB-amp agar plate. Incubate overnight at 37°C.
- Culture, isolate, and digest another 6-10 plasmid DNA recombinants. Determine correct recombinants by restriction endonuclease digests and electrophoresis.
- Transfect pcDNA3.1(+) human BCL-2 plasmid DNA into the P3-X63Ag8.653 cell line.
I. Transfection, Isolation, and Identification of BCL-2-Transfected Myeloma Cells
Solutions
- RIPA buffer: 150 mM NaCl, 50 mM Tris, 1% Igepal, 0.25% deoxycholate, pH 7.5. To make 1 liter, add 8.76g of sodium chloride, 6.35g Tris-HCl, 1.18g Tris base, 10ml Igepal, and 2.5 g deoxycholate to a total volume of 1 liter. Sterile filter in a Corning 0.2-µm vacuum filter storage unit and store at 4°C.
- Culturing medium for P3X63/Ag8.653 murine myeloma cells: Combine 890ml RPMI 1640, 100ml FBS, and 10 ml L-glutamine / pen-strep solution. Sterile filter in a Corning 0.2-µm vacuum filter storage unit and store media at 4°C.
- G418 selection medium, 1 liter, used for selection of BCL-2- transfected P3-X63Ag8.653 cells (parental cells P3- X63Ag8.653, ATCC CRL-1580): Combine 890ml RPMI 1640, 100 ml FBS, and 10 ml L-glutamine/pen-strep solution. Add geneticin (G418) from 50-mg/ml stock (potency is 600µg/mg) to final concentrations of 1 mg/ml, 500µg/ml, and 250µg/ml. Sterile filter in a Corning 0.2-µm vacuum filter storage unit and store media at 4°C.
- Culturing medium for BCL-2-transfected P3- X63Ag8.653 cells, 1 liter: Combine 890ml RPMI 1640, 100 ml FBS, 10 ml L-glutamine / pen-strep solution, and a final concentration of G418 at 200 µg/ml. Sterile filter in a Corning 0.2%tm vacuum filter storage unit. One week before using the cells for fusion, pass the cells into the just-described media without G418.
- Phosphate-buffered saline: 10× PBS from VWR (137mM NaCl, 2.7mM potassium chloride, 10mM phosphate buffer). To 100 ml of 10× PBS stock, add 900ml distilled water. Sterile filter in a Corning 0.2-µm vacuum filter storage unit.
- Hybridoma freezing medium: 90ml fetal bovine serum, 10 ml Hybri-Max DMSO, sterile filter, and store at 4°C.
- Alkaline phosphatase developing buffer, pH 9.5 (0.1 M Tris HCL, 0.1M NaCl, 5 mM MgCl2): 1.52 g Tris-HCl, 10.94g Tris-OH, 5.85g NaCl, 1.15g MgCl2.6H2O dissolved in 1 liter of distilled water, pH to 9.5, sterile filter, and store at 4°C.
- 5% PBST Blotto: To 100ml of 1× PBST (0.05% Tween-20), add 5 g powdered milk, mix well, and then store at 4°C.
- The day before transfection, plate P3X63/ Ag8.653 cells into 12 wells of a 24-well tissue culture plate at a density of 4 × 105 cells per well in 2ml of culturing media. Six of the wells will be used for transfection, and 6 wells will be used for controls for the G418 selection (see step 5). Incubate the cells overnight in a 37°C, 5% CO2 humidified incubator. The cells should be approximately 50% confluent.
- The DNA ratio used for transfection is 3:1. For each well, use 0.5 ml of serum-free RPMI 1640 medium containing 0.6µl of FuGENE 6 with 0.2µg of DNA encoding human BCL-2.
- The following steps are taken to prepare the FuGENE 6 reagent: DNA complex for transfection of P3X63/Ag8.653 murine myeloma with the human BCL-2 gene. Add 50µl of serum-free RPMI 1640 media to a small sterile tube. Add 3 µl of FuGENE 6 transfection reagent directly to the serum-free medium. Mix by tapping the tube gently. Add 0.2µg of the DNA in a volume of 1 µl to the tube. Mix the contents by tapping the tube gently (do not vortex). Incubate the reaction at room temperature for 15-30 min.
- Remove the medium from six wells of the plated P3X63/Ag8.653 cells. Add 0.6ml culture medium to each well. Using a sterile tip, add dropwise 53 µl of the complex mixture from step 3. Gently mix the cell suspension to disperse the reagent: DNA complex evenly. Return the cells to the incubator.
- Within 24h, carefully remove medium from wells that underwent transfection. To duplicate wells add 2ml of the 1-mg/ml, 500-µg/ml, and 250-µg/ml G418 selection media. To the remaining six wells that were not transfected, add to duplicate wells 2 ml of the 1-mg/ml, 500-µg/ml, and 250-µg/ml G418 selection media. These cells will serve as controls for the G418 selection process (cells should die).
- Within 4 days, remove selection media and replace wells with fresh selection media. As the selected cells grow, continue to maintain the cells in selection media. Change the media every 3 to 5 days.
- Expand the selected cell lines into T25-cm2 flasks in the respective G418 selection media. Grow the cells to 7-9 × 105/ml. Spin down the cells at 400g for 5 min. Decant the supernatant, resuspend the cells in hybridoma freezing medium, and aliquot 1 ml per 1.8- ml Nunc freezing vials. Place the cells into a styrofoam rack and then freeze overnight at -80°C. The next day, transfer the cells to LN2. Freeze back stocks of the transfected cell lines at 1 × 106 cells per vial in freezing media. Continue to maintain the cells in culture in respective selection media in order to prepare RIPA extracts to determine the presence of the human BCL- 2 protein (see later).
Steps
- Following the transfections and selection of P3- X63Ag8.653 cells with plasmid encoding human BCL-2, maintain the myeloma cells in G418 selection media containing final concentrations of 1 mg/ml, 500 µg/ml, and 250µg/ml in T25-cm2 flasks. Also maintain the parental P3-X63Ag8.653 cells, which will serve as negative controls.
- Count the parental P3-X63Ag8.653 and the BCL-2 transfected cell lines using trypan blue (see article by Hoffman). For each cell line, centrifuge 1 × 106 cells in a 15-ml conical tube at 400g for 5 min.
- Remove all of the culture supernatant. To make cell extracts in order to determine the presence of human BCL-2 by Western blot, add 10µl of chilled RIPA buffer to the cell pellet, resuspend the cells, and then transfer the respective cell suspensions to 1.5-ml Eppendorf tubes. Incubate tubes on ice for 15min.
- Microfuge the sample tubes in a microfuge set on high for 15min at 4°C. Remove the supernatant (discard the pellet) and then mix the supernatant 1:1 with 2× sample buffer. Heat samples at 96°C for 5 min.
- Load a 20-µl volume of the P3-X63Ag8.653 RIPA extract (parental cell line) and each P3XBCL-2- transfected cell extract onto individual lanes of a 1 × 10 well 8-16% Tris-glycine gel (InVitrogen) using one lane for SeeBlue Plus2 markers. Run the gel for 90min at 125 V and then transfer the gel to nitrocellulose. Block the nitrocelluose in 5% PBST Blotto overnight at 4°C.
- For detection of human BCL-2, add 10ml of antihuman BCL-2 (Dako). Dilute 160 µl of the antibody per 10ml in 5% PBST Blotto blocking buffer. Incubate the blot for 1 h at room temperature on a shaker or rocker platform.
- Wash the blot four times for 5min with PBST. Add 10ml of a 1:1000 dilution of goat anti-mouse IgGalkaline phosphatase-labeled conjugate diluted in 5% PBST Blotto blocking buffer to the blot. Incubate for 1 h on a rocker platform.
- Wash the blot four times with PBST. Develop the blot using 10ml alkaline phosphatase buffer containing 66 µl of NBT and 33 µl of BCIP. Immerse the blot into the developing substrate, place on a rocking platform, and incubate until a purple color develops. Stop the reaction by removing the developing substrate and rinsing the blot with ddH2O.
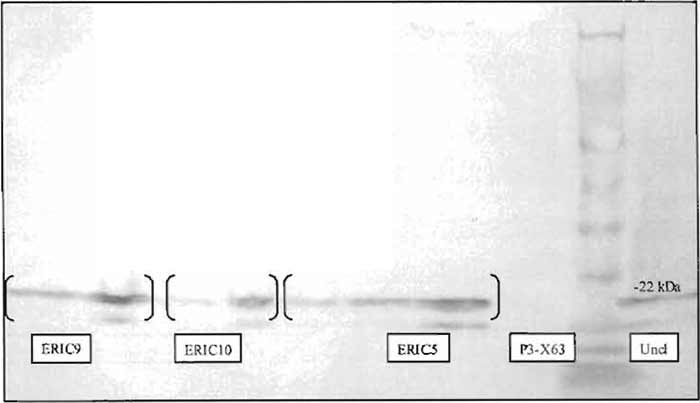
- Figure 2 demonstrates a Western blot indicating the presence of BCL-2 in RIPA extracts made from transfected, G418 selected P3-X63Ag8.653 myeloma cells.
- Expand the uncloned cells from wells that demonstrate the presence of BCL-2 into a 75-cm2 tissue culture flask containing 35 ml of media supplemented with 250µg/ml G418. Grow the cells to 7-9 × 105/ml. Spin down the cells at 400g for 5min. Decant the supernatant, resuspend the cells in hybridoma freezing media, and aliquot 1 ml per 1.8-ml Nunc freezing vials. Place the cells into a styrofoam rack and then freeze overnight at -80°C. The next day, transfer the cells to LN2.
 |
| FIGURE 2 Titration of RIPA extracts of P3-X63Ag8.653 clones (ERIC5, ERIC9, and ERIC10) stably expressing human BCL-2, as well as uncloned parental BCL-2 transfected cells, indicates the presence of human BCL-2 by Western blot using an anti-human bcl-2-specific monoclonal antibody. Control untransfected P3-X63Ag8.653 cells serve as a negative control. |
Steps
- Count the P3XBCL-2 cells (see article by Hoffman) and then dilute the cells to 330 cells in a total of 200ml of culturing media containing 250µg/ml G418. To increase the limit dilution cloning efficiency, the Origen cloning factor can be added to the G418 selection medium.
- Add 200µl of the cell suspension per well to 10 × 96-well sterile tissue culture plates. Incubate the cells at 37°C in a humidified incubator.
- Microscopically scan the wells to identify one cell per well within 48h of plating. Mark the wells that have clones derived from single cells.
- Once the clones have grown to cover 50-75% of the well, expand the cells into 24-well plates containing 2ml per well culturing media containing 200µg/ml G418. Allow the cells to grow to 80-90% confluency in 6 wells. Remove cells from 3 wells and make RIPA lysates using the aforementioned procedure. Test for expression of BCL-2 using the Western blotting procedure. (Fig. 2).
- Expand the cells that exhibit high BCL-2 expression levels to T25-cm2 tissue culture flasks. Freeze back master stocks as detailed earlier.
- Test the P3XBCL-2-transfected cells for their ability to negatively select (die) by placing the cells in fusion selection media containing HybriMax azaserine hypoxanthine. Due to the presence of BCL-2, the cells will take approximately 2-3 days longer to die as compared to the parental untransfected P3-X63Ag8.653 cell line.
Unfused B cells producing antigen-specific immunoglobulin can contribute to background, which may indicate false positives in a primary ELISA screen. It is imperative to change the media on fusion plates one to two times before screening (see Section IIIG, step 2).
References
Alligood, K. J., Milla, M., Rhodes, N., Ellis, B., Kilpatrick, K. E., Lee, A., Gilmer, T. J., and Lansing, T. L. (2000). Monoclonal antibodies generated against recombinant ATM support kinase activity. Hybridoma 19, 317-321.
Berek, C., Berger, A., and Apel, M. (1991). Maturation of the immune response in germinal centers. Cell 67, 1121-1129.
Bynum, J., Andrews, J. L., Ellis, B., Kull, E C., Austin, E. A., and Kilpatrick, K. E. (1999). Development of class-switched, affinity matured monoclonal antibodies following a 7 day immunization schedule. Hybridoma 18, 407-411.
Ellis, J. H., Ashman, C. A., Burden, M. N., Kilpatrick, K. E., Morse, M. A., and Hamblin, P. A. (2000). GRID, a novel Grg-2-related adapter protein which interacts with the activated T cell costimulatory receptor CD28. J. Immunol. 164, 5805-5814.
Ignar, D. M., Andrews, J. L., Witherspoon, S. M., Leray, J. D., Clay, W. C., Kilpatrick, K. E., Onori, J., Kost, T. A., and Emerson, D. L. (1998). Inhibition of establishment of primary and micrometastatic tumors by a urokinase plasminogen activator receptor antagonist. Clin. Exp. Metast. 16, 9-20.
Kelsoe, G. (1996) Life and death in germinal centers (Redux). Immunity 4, 107-111.
Kilpatrick, K. E., Cutler, T., Whitehorn, E., Drape, R. J., Macklin M. D., Witherspoon, S. M., Singer, S., and Hutchins, J. T. (1998). Gene gun delivered DNA-based immunizations mediate rapid production of murine monoclonal antibodies to the Flt-3 receptor. Hybridoma 17, 569-576.
Kilpatrick, K. E., Danger, D. P., Hull-Ryde, E. A., and Dallas, W. (2000). High affinity monoclonal antibodies generated in less than 30 days using 5 µg of DNA. Hybridoma 19, 297-302.
Kilpatrick, K. E., Kerner, S., Dixon, E. P., Hutchins, J. T., Parham, J. H., Condreay, J. P., and Pahel, G. (2002). In vivo expression of a GST-fusion protein mediates the rapid generation of affinity matured monoclonal antibodies using DNA-based immunizations. Hybridoma Hybridomi. 21, 237-243.
Kilpatrick, K., Sarzotti, M., and Kelsoe, G. (2003). Induction of B cells by DNA vaccines. In "DNA Vaccines" (H. C. J. Ertl, ed.), pp 66-81. Eurekah Publ.
Kilpatrick, K. E., Wring, S. A., Walker, D. H., Macklin, M. D., Payne, J. A., Su, J.-L., Champion, B. R., Caterson, B., and McIntyre, G. D. (1997). Rapid development of affinity matured monoclonal antibodies using RIMMS. Hybridoma 16, 381-389.
Kinch, K. C., Kilpatrick, K. E., Stewart, J. C., and Kinch, M. S. (2002). Antibody targeting of the EphA2 receptor tyrosine kinase on malignant carcinomas. Cancer Res. 62, 2840-2847.
Kroese, E G. M., Timens, W., and Nieuwenhuis, P. (1990). Germinal center reactions and B lymphocytes: Morphology and function. Curr. Topics. Pathol. 84, 103-148.
Levy, N. S., Malipiero, U. V., Lebecque, S. G., and Gearhart, P. J. (1989). Early onset of somatic mutations in immunoglobulin VH genes during the primary immune response. J. Exp. Med. 169, 2007-2019.
Lindley, K. M., Su, J.-L., Hodges, P. K., Wisely, C. B., Bledsoe, R. K., Condreay, J. P., Wineager, D. A., Hutchins, J. T., and Kost, T. A. (2000). Production of monoclonal antibodies using recombinant baculovirus displaying gp64-fusion proteins. J. Immunol. Methods 234, 123-135.
MacLennan, I. C. M. (1994). Germinal centers. Annu. Rev. Immunol. 12, 117-139.
Nossal, G. J. V. (1992). The molecular and cellular basis of affinity maturation in the antibody response. Cell 68, 1-2.
Wring, S. A., Kilpatrick, K. E., Hutchins, J. T., Witherspoon, S. M., Ellis, B., Jenner, W. N., and Serabjit-Singh, C. (1999). Shorter development of immunoassay for drugs: Application of the novel RIMMS technique enables rapid production of monoclonal antibodies to Ranitidine. J. Pharma. Biomed. Anal. 19, 695-707.




