Amino Acids and Proteins
Amino Acids and Proteins
Proteins are large, complex molecules composed of 20 commonly occurring amino acids (Figure 2-10). The amino acids are linked together by peptide bonds to form long, chainlike polymers. In the formation of a peptide bond, the carboxyl group of one amino acid is linked by a covalent bond to the amino group of another, with the elimination of water, as follows:
The combination of two amino acids by a peptide bond forms a dipeptide, and, as is evident, there is still a free amino group on one end and a free carboxyl group on the other; therefore, additional amino acids can be joined until a long chain is produced. The 20 different kinds of amino acids can be arranged in an enormous variety of sequences of up to several hundred amino acid units, accounting for the large diversity of proteins found among living organisms.
A protein is not just a long string of amino acids; it is a highly organized molecule. For convenience, biochemists recognize four levels of protein organization called primary, secondary, tertiary, and quaternary structures.
The primary structure of a protein constitutes the sequence of amino acids composing the polypeptide chain. Because the bonds between the amino acids in the chain can form only a limited number of stable angles, certain recurrent structural patterns are assumed by the chain. These bond angles give rise to the secondary structure, such as the alpha-helix, which makes helical turns in a clockwise direction like a screw (Figure 2- 11). The spirals of the chains are stabilized by hydrogen bonds, usually between a hydrogen atom of one amino acid and the peptide-bond oxygen of another amino acid from an adjacent turn of the helix. In addition, the helical and other configurations formed by the polypeptide chain themselves bend and fold, giving the protein its complex, yet stable, threedimensional tertiary structure (Figure 2-11). The folded chains are stabilized by the interactions between side groups of amino acids. One of these interactions is the disulfide bond, a covalent bond between the sulfur atoms in two cysteine amino acids that are brought together by folds in the polypeptide chain. Also stabilizing the tertiary structure of proteins are hydrogen bonds, ionic bonds, and hydrophobic bonds.
The term quaternary structure describes proteins that contain more than one polypeptide chain. For example, hemoglobin (the oxygen-carrying substance in blood) of higher vertebrates is composed of four polypeptide subunits held together in a single protein molecule (Figure 2-11).
Proteins perform many functions in living organisms. They serve as the structural framework of protoplasm and form many cellular components. Proteins also may function as enzymes, the biological catalysts required for almost every reaction in the body. Enzymes lower the activation energy required for specific reactions and enable life processes to proceed at moderate temperatures. They control the reactions by which food is digested, absorbed, and metabolized. They promote the synthesis of structural materials for growth and to replace those lost by wear on the body. They determine the release of energy used in respiration, growth, muscle contraction, physical and mental activities, and many other activities. Enzyme action is described in Cellular Metabolism.
Proteins are large, complex molecules composed of 20 commonly occurring amino acids (Figure 2-10). The amino acids are linked together by peptide bonds to form long, chainlike polymers. In the formation of a peptide bond, the carboxyl group of one amino acid is linked by a covalent bond to the amino group of another, with the elimination of water, as follows:
The combination of two amino acids by a peptide bond forms a dipeptide, and, as is evident, there is still a free amino group on one end and a free carboxyl group on the other; therefore, additional amino acids can be joined until a long chain is produced. The 20 different kinds of amino acids can be arranged in an enormous variety of sequences of up to several hundred amino acid units, accounting for the large diversity of proteins found among living organisms.
A protein is not just a long string of amino acids; it is a highly organized molecule. For convenience, biochemists recognize four levels of protein organization called primary, secondary, tertiary, and quaternary structures.
The primary structure of a protein constitutes the sequence of amino acids composing the polypeptide chain. Because the bonds between the amino acids in the chain can form only a limited number of stable angles, certain recurrent structural patterns are assumed by the chain. These bond angles give rise to the secondary structure, such as the alpha-helix, which makes helical turns in a clockwise direction like a screw (Figure 2- 11). The spirals of the chains are stabilized by hydrogen bonds, usually between a hydrogen atom of one amino acid and the peptide-bond oxygen of another amino acid from an adjacent turn of the helix. In addition, the helical and other configurations formed by the polypeptide chain themselves bend and fold, giving the protein its complex, yet stable, threedimensional tertiary structure (Figure 2-11). The folded chains are stabilized by the interactions between side groups of amino acids. One of these interactions is the disulfide bond, a covalent bond between the sulfur atoms in two cysteine amino acids that are brought together by folds in the polypeptide chain. Also stabilizing the tertiary structure of proteins are hydrogen bonds, ionic bonds, and hydrophobic bonds.
The term quaternary structure describes proteins that contain more than one polypeptide chain. For example, hemoglobin (the oxygen-carrying substance in blood) of higher vertebrates is composed of four polypeptide subunits held together in a single protein molecule (Figure 2-11).
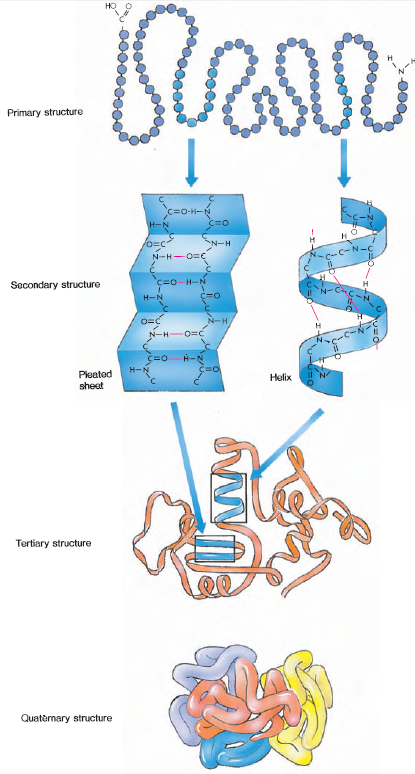 |
| Figure 2-11 Structure of proteins. The amino acid sequence of a protein (primary structure) encourages the formation of hydrogen bonds between nearby amino acids, producing coils and foldbacks (the secondary structure). Bends and helices cause the chain to fold back on itself in a complex manner (tertiary structure). Individual polypeptide chains of some proteins aggregate together to form the functional molecule composed of several subunits (quaternary structure). |
Proteins perform many functions in living organisms. They serve as the structural framework of protoplasm and form many cellular components. Proteins also may function as enzymes, the biological catalysts required for almost every reaction in the body. Enzymes lower the activation energy required for specific reactions and enable life processes to proceed at moderate temperatures. They control the reactions by which food is digested, absorbed, and metabolized. They promote the synthesis of structural materials for growth and to replace those lost by wear on the body. They determine the release of energy used in respiration, growth, muscle contraction, physical and mental activities, and many other activities. Enzyme action is described in Cellular Metabolism.




