Carbohydrates: Nature’s Most Abundant Organic Substance
Carbohydrates: Nature’s
Most Abundant Organic
Substance
Carbohydrates are compounds of carbon,
hydrogen, and oxygen. They are
usually present in the ratio of 1 C: 2 H:
1O and are grouped as H—C—OH.
Carbohydrates function in protoplasm
mainly as structural elements and as a
source of chemical energy. Glucose is
the most important of these energystoring
carbohydrates. Familiar examples
of carbohydrates include sugars,
starches, and cellulose (the woody
structure of plants). Cellulose occurs
on earth in greater quantities than all
other organic materials combined. Carbohydrates
are synthesized by green
plants from water and carbon dioxide,
with the aid of solar energy. This
process, called photosynthesis, is a
reaction upon which all life depends,
for it is the starting point in the formation
of food.
Carbohydrates are usually categorized into the following three classes: (1) monosaccharides, or simple sugars; (2) disaccharides, or double sugars; and (3) polysaccharides, or complex sugars. Simple sugars are composed of carbon chains containing 4 carbons (tetroses), 5 carbons (pentoses), or 6 carbons (hexoses). Other simple sugars may have up to 10 carbons, but these sugars are not biologically important.
Simple sugars, such as
glucose, galactose, and fructose, all
contain a free sugar group, in which the double-bonded O may
be attached to the terminal or nonterminal
carbons of a chain. The hexose glucose (also called dextrose) is particularly
important to the living world.
Glucose is often shown as a straight
chain (Figure 2-2A), but in water it
tends to form a cyclic compound
(Figure 2-2B). The “chair” diagram
(Figure 2-3) of glucose best represents
its true configuration, but all forms of
glucose, however represented, are the
same molecule. Other hexoses of biological
significance include galactose
and fructose, which are compared
with glucose in Figure 2-4.
Disaccharides are double sugars
formed by the bonding of two simple
sugars. An example is maltose (malt
sugar), composed of two glucose molecules.
As shown in Figure 2-5, the
two glucose molecules are condensed
together by the removal of a molecule
of water. This condensation reaction,
with the sharing of an oxygen atom by
the two sugars, characterizes the formation
of all disaccharides. Two other
common disaccharides are sucrose
(ordinary cane, or table, sugar),
formed by the linkage of glucose and
fructose, and lactose (milk sugar),
composed of glucose and galactose.
Polysaccharides are composed of many molecules of simple sugars (usually glucose) linked together in long chains called polymers. Their empirical formula is usually written (C6H10O5)n, where n designates the number of simple sugar subunits contained in the polymer. Starch is the common form in which sugar is stored in most plants and is an important food for animals. Glycogen is an important form for storing sugar in animals. It is found mainly in liver and muscle cells in vertebrates. When needed, glycogen is converted to glucose and delivered by the blood to the tissues. Another polymer is cellulose, the principal structural carbohydrate of plants.
Lipids: Fuel Storage and Building Material
Lipids are fats and fatlike substances. They are composed of molecules of low polarity; consequently, they are virtually insoluble in water but are soluble in organic solvents, such as acetone and ether. The three principal groups of lipids are neutral fats, phospholipids, and steroids.
Neutral Fats
The neutral or “true” fats are major fuels of animals. Stored fat may be derived directly from dietary fat or indirectly from dietary carbohydrates that are converted to fat for storage. Fats are oxidized and released into the bloodstream as needed to meet tissue demands, especially those of active muscle.
Neutral fats include triglycerides, which are molecules consisting of glycerol and three molecules of fatty acids. Neutral fats are therefore esters, a combination of an alcohol (glycerol) and an acid. Fatty acids in triglycerides are simply long-chain monocarboxylic acids; they vary in size but are commonly 14 to 24 carbons long. Production of a typical fat by the union of glycerol and stearic acid is shown in Figure 2-6A. In this reaction, three fatty-acid molecules can be seen to have united with OH groups of the glycerol to form stearin (a neutral fat) plus three molecules of water.
Most triglycerides contain two or
three different fatty acids attached to
glycerol, and bear ponderous names
such as myristoyl stearoyl glycerol
(Figure 2-6B). The fatty acids in this
triglyceride are saturated; every carbon
within the chain holds two hydrogen
atoms. Saturated fats, more common
in animals than in plants, are
usually solid at room temperature. Unsaturated fatty acids, typical of
plant oils, have two or more carbon
atoms joined
by double bonds; the carbons
are not “saturated” with hydrogen
atoms and are able to form bonds with
other atoms. Two common unsaturated
fatty acids are oleic acid and linoleic
acid (Figure 2-7). Plant fats such as
peanut oil and corn oil tend to be liquid
at room temperature.
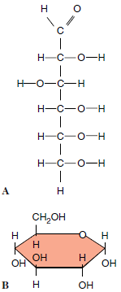 |
| Figure 2-2 Two ways of depicting the structural formula of the simple sugar glucose. In A, the carbon atoms are shown in open- chain form. When dissolved in water, glucose tends to assume a ring form as in B. In this ring model the carbon atoms located at each turn in the ring are usually not shown. |
Carbohydrates are usually categorized into the following three classes: (1) monosaccharides, or simple sugars; (2) disaccharides, or double sugars; and (3) polysaccharides, or complex sugars. Simple sugars are composed of carbon chains containing 4 carbons (tetroses), 5 carbons (pentoses), or 6 carbons (hexoses). Other simple sugars may have up to 10 carbons, but these sugars are not biologically important.
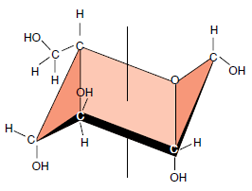 |
| Figure 2-3 “Chair” representation of a glucose molecule. |
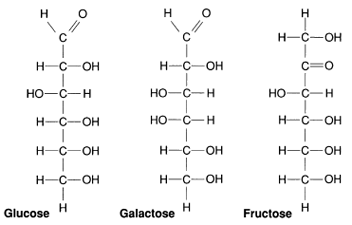 |
| Figure 2-4 These three hexoses are the most common monosaccharides. |
Polysaccharides are composed of many molecules of simple sugars (usually glucose) linked together in long chains called polymers. Their empirical formula is usually written (C6H10O5)n, where n designates the number of simple sugar subunits contained in the polymer. Starch is the common form in which sugar is stored in most plants and is an important food for animals. Glycogen is an important form for storing sugar in animals. It is found mainly in liver and muscle cells in vertebrates. When needed, glycogen is converted to glucose and delivered by the blood to the tissues. Another polymer is cellulose, the principal structural carbohydrate of plants.
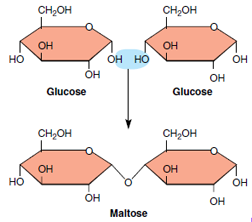 |
| Figure 2-5 Formation of a double sugar (disaccharide maltose) from two glucose molecules with the removal of one molecule of water. |
Lipids: Fuel Storage and Building Material
Lipids are fats and fatlike substances. They are composed of molecules of low polarity; consequently, they are virtually insoluble in water but are soluble in organic solvents, such as acetone and ether. The three principal groups of lipids are neutral fats, phospholipids, and steroids.
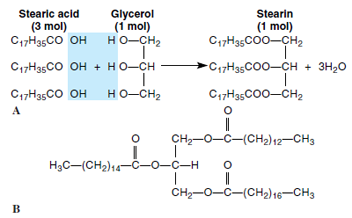 |
| Figure 2-6 Neutral fats. A, Formation of a neutral fat from three molecules of stearic acid (a fatty acid) and glycerol. B, A neutral fat bearing three different fatty acids. |
The neutral or “true” fats are major fuels of animals. Stored fat may be derived directly from dietary fat or indirectly from dietary carbohydrates that are converted to fat for storage. Fats are oxidized and released into the bloodstream as needed to meet tissue demands, especially those of active muscle.
Neutral fats include triglycerides, which are molecules consisting of glycerol and three molecules of fatty acids. Neutral fats are therefore esters, a combination of an alcohol (glycerol) and an acid. Fatty acids in triglycerides are simply long-chain monocarboxylic acids; they vary in size but are commonly 14 to 24 carbons long. Production of a typical fat by the union of glycerol and stearic acid is shown in Figure 2-6A. In this reaction, three fatty-acid molecules can be seen to have united with OH groups of the glycerol to form stearin (a neutral fat) plus three molecules of water.
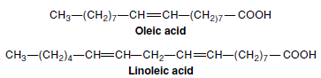 |
| Figure 2-7 Unsaturated fatty acids: oleic acid having one double bond and linoleic acid having two double bonds. The remainder of the hydrocarbon chains of both acids is saturated. |
 |




