Prebiotic Synthesis of Small Organic Molecules
Prebiotic Synthesis of
Small Organic Molecules
The Oparin-Haldane hypothesis stimulated
experimental work to test the
hypothesis that organic compounds
characteristic of life could be formed
from the simpler molecules present in
the prebiotic environment. In 1953,
Stanley Miller and Harold Urey in
Chicago successfully simulated the
conditions thought to prevail on the
primitive earth. Miller built an apparatus
designed to circulate a mixture of
methane, hydrogen, ammonia, and water
past an electric spark (Figure 2-12).
Water in the flask was boiled to produce
steam that helped to circulate
the gases. The products formed in the
electrical discharge (representing lightning)
were condensed in the condenser
and collected in the U-tube and
small flask (representing an ocean).
After a week of continuous sparking, approximately 15% of the carbon that was originally in the reducing “atmosphere” had been converted into organic compounds that collected in the “ocean.” The most striking finding was that many compounds related to life were synthesized. These compounds included four of the amino acids commonly found in proteins, urea, and several simple fatty acids. We can appreciate the astonishing nature of this synthesis when we consider that there are thousands of known organic compounds with structures no more complex than those of the amino acids formed. Yet in Miller’s synthesis, most of the relatively few substances formed were compounds found in living organisms. This result was surely no coincidence, and it suggests that prebiotic synthesis on the primitive earth may have occurred under conditions not greatly different from those that Miller chose to simulate.
Miller’s experiments have been criticized in light of current opinion that the early atmosphere on earth was quite different from Miller’s strongly reducing simulated atmosphere. Nevertheless, Miller’s work stimulated many other investigators to repeat and extend his experiment. Amino acids were found to be synthesized in many different kinds of gas mixtures that were heated (volcanic heat), irradiated with ultraviolet light (solar radiation), or subjected to electrical discharge (lightning). The only conditions required to produce amino acids were that the gas mixture be reducing and that it be subjected violently to a source of energy. In other experiments, electrical discharges were passed through mixtures of carbon monoxide, nitrogen, and water, yielding amino acids and nitrogenous bases. Although reaction rates were much slower than in atmospheres containing methane and ammonia, and yields were poor in comparison, these experiments support the hypothesis that the chemical beginnings of life can occur in atmospheres that are only mildly reducing. The need for methane and ammonia, however, led to proposals that these substances might have been introduced by comets or meteorites, or that they were synthesized near the hydrothermal vents.
Thus the experiments of many scientists have shown that highly reactive intermediate molecules such as hydrogen cyanide, formaldehyde, and cyanoacetylene are formed when a reducing mixture of gases is subjected to a violent energy source. These molecules react with water and ammonia or nitrogen to form more complex organic molecules, including amino acids, fatty acids, urea, aldehydes, sugars, and nitrogenous bases (purines and pyrimidines), all of the building blocks required for the synthesis of the most complex organic compounds of living matter.
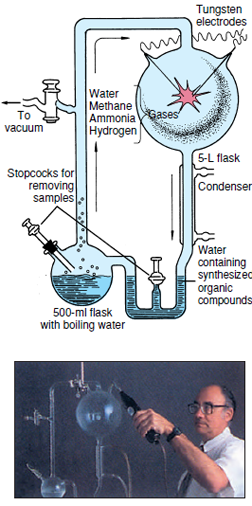 |
| Figure 2-12 Dr. S. L. Miller with a replica of the apparatus used in his 1953 experiment on the synthesis of amino acids with an electric spark in a strongly reducing atmosphere. |
After a week of continuous sparking, approximately 15% of the carbon that was originally in the reducing “atmosphere” had been converted into organic compounds that collected in the “ocean.” The most striking finding was that many compounds related to life were synthesized. These compounds included four of the amino acids commonly found in proteins, urea, and several simple fatty acids. We can appreciate the astonishing nature of this synthesis when we consider that there are thousands of known organic compounds with structures no more complex than those of the amino acids formed. Yet in Miller’s synthesis, most of the relatively few substances formed were compounds found in living organisms. This result was surely no coincidence, and it suggests that prebiotic synthesis on the primitive earth may have occurred under conditions not greatly different from those that Miller chose to simulate.
Miller’s experiments have been criticized in light of current opinion that the early atmosphere on earth was quite different from Miller’s strongly reducing simulated atmosphere. Nevertheless, Miller’s work stimulated many other investigators to repeat and extend his experiment. Amino acids were found to be synthesized in many different kinds of gas mixtures that were heated (volcanic heat), irradiated with ultraviolet light (solar radiation), or subjected to electrical discharge (lightning). The only conditions required to produce amino acids were that the gas mixture be reducing and that it be subjected violently to a source of energy. In other experiments, electrical discharges were passed through mixtures of carbon monoxide, nitrogen, and water, yielding amino acids and nitrogenous bases. Although reaction rates were much slower than in atmospheres containing methane and ammonia, and yields were poor in comparison, these experiments support the hypothesis that the chemical beginnings of life can occur in atmospheres that are only mildly reducing. The need for methane and ammonia, however, led to proposals that these substances might have been introduced by comets or meteorites, or that they were synthesized near the hydrothermal vents.
Thus the experiments of many scientists have shown that highly reactive intermediate molecules such as hydrogen cyanide, formaldehyde, and cyanoacetylene are formed when a reducing mixture of gases is subjected to a violent energy source. These molecules react with water and ammonia or nitrogen to form more complex organic molecules, including amino acids, fatty acids, urea, aldehydes, sugars, and nitrogenous bases (purines and pyrimidines), all of the building blocks required for the synthesis of the most complex organic compounds of living matter.




