Appearance of Photosynthesis and Oxidative Metabolism
Appearance of
Photosynthesis and
Oxidative Metabolism
Autotrophy evolved in the form of photosynthesis. In photosynthesis, hydrogen atoms obtained from water react with carbon dioxide obtained from the atmosphere to generate sugars and molecular oxygen.
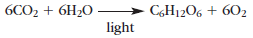
The sugars provide nutrition to the organism and molecular oxygen is released into the atmosphere. This equation summarizes the many reactions now known to occur in the process of photosynthesis. Undoubtedly these reactions did not appear all at once, and other reduced compounds, such as hydrogen sulfide (H2S), probably were the early sources of hydrogen.
Gradually, oxygen produced by photosynthesis accumulated in the atmosphere. When atmospheric oxygen reached approximately 1% of its current level, ozone began to accumulate and to absorb ultraviolet radiation, thereby greatly restricting the amount of ultraviolet light that reached the earth. Land and surface waters then were occupied by photosynthetic organisms, thereby increasing oxygen production.
Accumulation of atmospheric oxygen would interfere with anaerobic cellular metabolism that had evolved in the primitive reducing atmosphere. As the atmosphere slowly changed from a somewhat reducing to a highly oxidizing one, a new and highly efficient kind of metabolism appeared: oxidative (aerobic) metabolism. By using available oxygen as a terminal electron acceptor and completely oxidizing glucose to carbon dioxide and water, much of the bond energy stored by photosynthesis could be recovered. Most living forms became completely dependent upon oxidative metabolism.
Our atmosphere today is strongly oxidizing. It contains 78% molecular nitrogen, approximately 21% free oxygen, 1% argon, and 0.03% carbon dioxide. Although the time course for production of atmospheric oxygen is much debated, the most important source of oxygen is photosynthesis. Almost all oxygen currently produced comes from cyanobacteria (blue-green algae), eukaryotic algae, and plants. Each day these organisms combine approximately 400 million tons of carbon dioxide with 70 million tons of hydrogen to produce 1.1 billion tons of oxygen. Oceans are a major source of oxygen. Almost all oxygen produced today is consumed by organisms for respiration; otherwise, the amount of oxygen in the atmosphere would double in approximately 3000 years. Because Precambrian fossil cyanobacteria resemble modern cyanobacteria, it is reasonable to suppose that oxygen entering the early atmosphere came from their photosynthesis.
Autotrophy evolved in the form of photosynthesis. In photosynthesis, hydrogen atoms obtained from water react with carbon dioxide obtained from the atmosphere to generate sugars and molecular oxygen.
The sugars provide nutrition to the organism and molecular oxygen is released into the atmosphere. This equation summarizes the many reactions now known to occur in the process of photosynthesis. Undoubtedly these reactions did not appear all at once, and other reduced compounds, such as hydrogen sulfide (H2S), probably were the early sources of hydrogen.
Gradually, oxygen produced by photosynthesis accumulated in the atmosphere. When atmospheric oxygen reached approximately 1% of its current level, ozone began to accumulate and to absorb ultraviolet radiation, thereby greatly restricting the amount of ultraviolet light that reached the earth. Land and surface waters then were occupied by photosynthetic organisms, thereby increasing oxygen production.
Accumulation of atmospheric oxygen would interfere with anaerobic cellular metabolism that had evolved in the primitive reducing atmosphere. As the atmosphere slowly changed from a somewhat reducing to a highly oxidizing one, a new and highly efficient kind of metabolism appeared: oxidative (aerobic) metabolism. By using available oxygen as a terminal electron acceptor and completely oxidizing glucose to carbon dioxide and water, much of the bond energy stored by photosynthesis could be recovered. Most living forms became completely dependent upon oxidative metabolism.
Our atmosphere today is strongly oxidizing. It contains 78% molecular nitrogen, approximately 21% free oxygen, 1% argon, and 0.03% carbon dioxide. Although the time course for production of atmospheric oxygen is much debated, the most important source of oxygen is photosynthesis. Almost all oxygen currently produced comes from cyanobacteria (blue-green algae), eukaryotic algae, and plants. Each day these organisms combine approximately 400 million tons of carbon dioxide with 70 million tons of hydrogen to produce 1.1 billion tons of oxygen. Oceans are a major source of oxygen. Almost all oxygen produced today is consumed by organisms for respiration; otherwise, the amount of oxygen in the atmosphere would double in approximately 3000 years. Because Precambrian fossil cyanobacteria resemble modern cyanobacteria, it is reasonable to suppose that oxygen entering the early atmosphere came from their photosynthesis.




