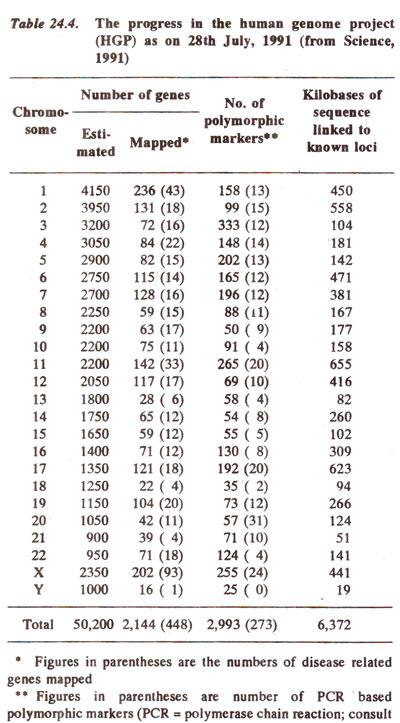
Chromosome mapping in humans (including RFLPs, etc.)
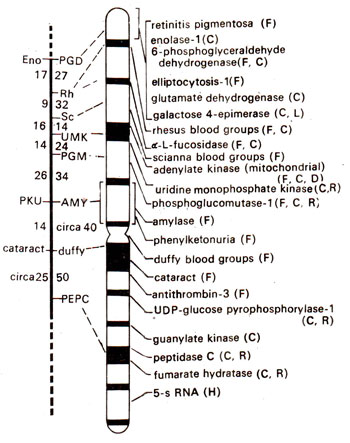
Fig. 24.6. Map of chromosome-1 of the human genome and the genes that have been identified. In the figure on the left, linkage group is shown with location of genes and the recombination frequencies (figures on left are from male and those on right from female meiosis; note higher frequencies in female). Broken line shows terminal region where no genes could be located till 1983. In the figure on right G bands, centromere and location of different genes are shown. Methods used for chromosome mapping are shown in parentheses (F = family linkage; C = cell hybrids; R = radiation induced loss; LD = linkage disequilibrium with another marker; H = in situ hybridization; D = deletion mapping (redrawn from Fincham-Genetics, 1983; current map will be much more extensive; see Table 24.4)
If cells (mouse cell line) resistant to a toxic drug like bromodeoxyuridine are hybridized with normal human cells, the derived hybrid cells on repeated divisions in HAT medium lose all other human chromosomes except the one responsible for synthesis of thymidine kinase. The growth of cells on HAT medium takes place only when these cells synthesize thymidine kinase, besides other enzymes. This exceptional human chromosome was always found to be the same i.e. chromosome 17 and could be identified by banding technique. The gene for thymidine kinase could thus be located on chromosome 17 of humans.
Using resistant lines for several markers,independence or linkage between several genes could be worked out. Differences between human and mouse enzymes could also be worked out and a number of such enzymes could be identified. In these cases absence or presence of enzyme was not used, but instead migration of enzyme on electrophoretic gel, was used, as a criterion.
By studying cell lines, which have lost only a part of a chromosome rather than the whole chromosome, genes could also be located on a specific region of a chromosome. The progress made in chromosome mapping in humans upto the year 1991 is summarized in Table 24.4. The progress in mapping of the longest human chromosome called chromosome-1 is shown in Figure 24.6. It is hoped that the human gene map should be completely defined by the end of present century, if not at the chromosomal level, then at the genetic level.

Fig. 24.6. Map of chromosome-1 of the human genome and the genes that have been identified. In the figure on the left, linkage group is shown with location of genes and the recombination frequencies (figures on left are from male and those on right from female meiosis; note higher frequencies in female). Broken line shows terminal region where no genes could be located till 1983. In the figure on right G bands, centromere and location of different genes are shown. Methods used for chromosome mapping are shown in parentheses (F = family linkage; C = cell hybrids; R = radiation induced loss; LD = linkage disequilibrium with another marker; H = in situ hybridization; D = deletion mapping (redrawn from Fincham-Genetics, 1983; current map will be much more extensive; see Table 24.4)
** Figures in parentheses are number of PCR based polymorphic markers (PCR = polymerase chain reaction; consult Genetic Engineering and Biotechnology 1. Recombinant DNA and PCR (Cloning and Amplification of DNA)).
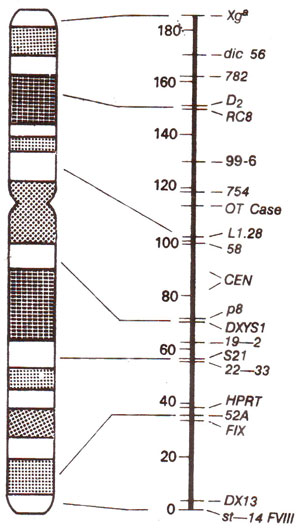
Fig. 24.7. Genetic map of human X-chromosome; in the figure on the left, the G-bands of the X-chromosome are shown; in the figure on the right, linkage map is shown with distances (recombination frequencies as centimorgan or cM units) on one side and location of genes on the other side.
The linkage map of X-chromosome was prepared by first locating DNA markers using either a physical method due to in situ hybridization, or biochemical meth. j where marker DNA was hybridized on the DNA of individual hybrid cell lines, each containing a different portion of human X-chromosome. In the later case, absence of hybridization was used to infer that the marker is located on the missing segment of X-chromosome.
Gene distances and linear order of genes were determined by analysing families with the help of large number of DNA markers. The basis for using families was as follows. The X-chromosome from a maternal grandfather, without undergoing any recombination passes to the mother, where it undergoes recombination to be identified in children (boys and girls). Recombinant and non-recombinant chromosomes can be scored in children by DNA markers. From the data, gene distances and linear order of genes can be established. In a study conducted recently, genetic linkage relationships in X-chromosome were established among 21 DNA markers by examining DNA from 38 normal families each involving maternal and paternal grandparents, parents and children (an average of 9 children). As a result of this study, a linkage map of human X-chromosome was prepared which is reproduced in Figure 24.7.
In recent years RFLPs have been used as molecular genetic markers in human beings (see Genetic Engineering and Biotechnology 2. Restriction Maps and Molecular Genetic Maps). These RFLPs were revealed using a number of restriction endonucleases for digestion of genomic DNA, followed by hybridization with specific genomic or cDNA clones (see Genetic Engineering and Biotechnology 1. Recombinant DNA and PCR (Cloning and Amplification of DNA)) used as probes. The technique used for RFLP mapping in humans differs from those in mice, fruitfly or plants. Segregation patterns of the markers is studied within a panel of reference families (cell lines from 59 such families as a source of DNA have been maintained in Paris) and recombination frequencies calculated from this finite and limited sample size. Once RFLPs are ordered, models are fitted by maximizing the likelihood of parameters. The likelihoods of different models are compared by comparing likelihood ratios (1000 : 1, 100 : 1 or 10 : 1). The log10 of this ratio is called LOD score (for 1000 : 1, LOD score = 3, for 100 : 1, LOD score = 2), which is used for linkage analysis. A LOD score equal to or exceeding 3 is a proof of linkage. Of 7000-8000 loci needed for a saturated map with a resolution of 1 cM, about 3000 loci have already been mapped (Table 24.4; for more details consult an advanced book on RFLPs or a recent book on human genetics).

Fig. 24.7. Genetic map of human X-chromosome; in the figure on the left, the G-bands of the X-chromosome are shown; in the figure on the right, linkage map is shown with distances (recombination frequencies as centimorgan or cM units) on one side and location of genes on the other side.
Earlier during the years 1986 and 87, there has been a debate among the molecular biologists about the desirability and feasibility of sequencing the nucleotides in the entire human genome. This will involve sequencing of 3 billion nucleotides and will need 30,000 person-years (expenses more than two billion U.S. dollars). The idea later gained momentum and the merits of sucha project were recognized. A part of the jenome has already been sequenced and the next few years will see the sequencing of the whole human genome (Table 24.5).