Nuclear pore occupies a central position among the major cellular structures, but still remains one of the least understood structures. However, during the late 1980s and early 1990s, significant progress has been made towards a better understanding of the structure and function of the nuclear pore. During this recent past, new pore proteins have been identified (particularly in yeast), the genes for several of these proteins have been cloned, a number of mutants in these pore proteins have been isolated and detailed mechanism of nucleocytoplasmic traffic has been proposed. Further, the pore has been reconstituted
in vitro, a number of
'signal sequences' and one or more
'signal sequence receptors' have been identified, and a new
'basket-like structure' has been found attached to the inner side of the nuclear pore. (Consult review by Douglass J. Forbes in
Ann. Rev. Cell BioL, 1992 for detailed account).
Structure of nuclear pore complex. The nuclear pore is a large complex structure of 124 million daltons or 30 times the size of a eukaryotic ribosome. The pore is 120nm in diameter and 50nm in thickness. It consists of four separate elements : (i) the
scaffold, which includes majority of the pore, (ii) the central hub or
transporter, which carries out active transport (both import and export) of proteins and RNAs, (iii) short
thick filaments attached to the cytoplasmic side of the pore and (iv) a newly discovered
basket attached to the nucleoplasmic side of the pore.
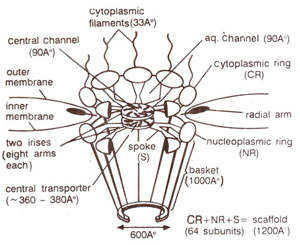
Fig. 6.4. Detailed structure of the nuclear pore, showing iris and transporter.
The scaffold is a stack of three closely apposed rings, namely the
cytoplasmic ring, the
nucleoplasmic ring and a
central ring of thick spokes. Each ring has a eightfold symmetry. The spokes of central ring are attached to the transporter on the inner side, and to the nucleoplasmic and cytoplasmic rings on the outer side. Interspersed between the spokes are aqueous channels, 9nm wide, which allow diffusion of proteins and metabolites between the nucleus and the cytoplasm.
The transporter is a proteinaceous ring, 36-38nm in diameter and consists of two
irises of eight arms each. The two irises are assumed to be stacked atop one another and open sequentially, each like the diaphragm of a camera, to let a nuclear protein or RNA to pass through from the nucleus to the cytoplasm. On the cytoplasmic side of the pore, thick fibres (3.3nm in diameter) extend into the cytoplasm. On the nuclear side, a large basket like structure is found, which consists of eight filaments (each lOOnm long), extending from nucleoplasmic ring of the pore and meeting a smaller ring (60nm in diameter) within the nucleus. This basket may play an important role in RNA export. The detailed structure of nuclear pore complex is shown in Figure 6.4.

Fig. 6.4. Detailed structure of the nuclear pore, showing iris and transporter.
Role of nuclear pore in transport. The import and export of protejns and RNAs into and outside the nucleus are facilitated by the presence of
signal sequences. Even gold particles 25nm in diameter, if coated with a signal sequence—bearing nuclear protein, are transported into and outside the nucleus. ATP has also been found to be essential for nuclear transport. The transport (this is usually unidirectional) actually involves following two steps: (i) an ATP-independent, but signal-sequence dependent step involving binding of
protein to the pore, and (ii) an ATP-requiring step, involving translocation through the pore; this step is
the only energy requiring step. It has been shown that in the absence of ATP, the protein binds to the pore, but can not be transported to the nucleus.
(a) Protein import and export Nuclear proteins, like other proteins, are synthesized in the cytoplasm,
and are later imported into the nucleus. Mostly, these proteins are transported unidirectionally from the cytoplasm to the nucleus
and can
not exit the nuclear pore, once they are imported. Such proteins include SV40
T antigen and
Xenopus nucleoplasmin, which have been studied in
some detail. There are few other proteins,
which shuttle between nucleus and cytoplasm,
either constitutively (irrespective of requirement)
or in response to regulatory signals. These include various nuclear proteins like
hsc70 and the cAMP dependent protein
kinase. It is predicted that these proteins contain an additional signal sequence for protein export. Some of the ribosomai proteins are imported to associate with rRNA, so that the
ribosomai subunits are assembled inside the nucleus and then exported through the pore to the cytoplasm, a process, which is ATP-dependent.
Although several signal sequences for import have
been identified, no signal sequences for export are
known. These should be discovered only in future.
Signal sequences that have been identified for
many nuclear proteins to be imported, have often
been compared with SV40 T antigen sequence, Pro-Lys-Lys-Lys-Arg-Lys-Val. In this sequence, mutation of second lysine to threonine greatly reduces import, suggesting the role of this amino acid in import. Many other signal sequences resemble more closely, the first identified signal sequence, i.e.
Xenopus protein
nucleoplasmin, whose gene has also been cloned now. The signal sequence of nucleoplasmin is larger (16 amino acids) than that for T-antigen,and has two independent basic domains separated by 10 spacer amino acids. It is now believed that the bipartite nucleoplasmin signal sequence will be the type most often found in other proteins.
The existence of nuclear signal sequences predicts that there must also exist one or more receptors for these signals. Some such receptors have been isolated (particularly in yeast) and are believed to be located either in the pore or in the cytoplasm. In the latter case, they bind the signal sequence and carry the protein to the pore.
(b) RNA export and import. One of the most vital roles of nuclear pore is the unidirectional export of RNA, including
tRNAs, snRNAs (small nuclear RNAs) and
processed mRNA. Ribosomai RNA is used in the nucleus for assembly of small subunits,. which are later exported, as discussed above. Thus, the rRNA and unprocessed mRNA are not allowed to be exported. It has been shown that for export of processed mRNAs and snRNAs, the
5' monomethyl cap acquired during transcription is essential (see
Expression of Gene : Protein Synthesis 3. RNA Processing (RNA Splicing, RNA Editing and Ribozymes)). Several cap binding proteins have been identified and may help in RNA export. They may work as RNA export receptors or may contain protein export signal, which helps the export of
cap-binding protein-mRNA complex.
Few cellular RNAs are also imported into the nucleus. These include
snRNAs (particularly Ul and U2), which are synthesized with monomethyl cap and are first exported to the cytoplasm, to be converted into dimethyl form, which is assembled as snRNPs (small nuclear ribonucleo-proteins). These snRNPs, with the help of a bipartite signal (one for export and other for import), are reimported into the nucleus. T-DNA of Ti plasmid of
Agrobacterium tumefaciens (used for gene transfer; see
Genetic Engineering and Biotechnology 4. Gene Transfer Methods and Transgenic Organisms) is an example of the import of DNA into the nucleus.





