Varanus Albigularis
White-throated monitor, Rock leguaan, Tree leguaan.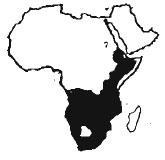 |
Varanus albigularis albigularis Daudin 1802
Varanus albigularis angolensis Schmidt 1933
Varanus albigularis microstictus Boett ger 1893
The white-throated monitor is a big, sturdy lizard found in much of central and southern Africa; Central African Republic, Sudan, Djibouti, Ethiopia, Somalia, Gabon, Congo Republic, Zaire, Uganda, Rwanda, Burundi, Kenya, Tanzania, Angola, Zambia, Malawi, Zimbabwe, Mozumbique, Namibia, Botswana and South Africa. The species is apparently extinct in Lesotho (Luxmoore et al 1988 and references therein). V.ocellatus (Heyden in Ruppell 1830) is recorded from southern Egypt and neighbouring countries and is probably closely related to V.exanthimaticus (Anderson 1898, Schmidt 1919. Hafez1978- see under V.exanthematicus). This monitor is found in a variety of dry habitats including steppes. prairies and savannahs but are absent from desert interiors, rainforests and thick scrub forests. Unlike Nile monitors, they appear to shun human settlements.
These lizards were previously known as subspecies of V.exanthematicus, but have been separated on the basis of differences in hemipenal morphology (Bohme 1988, 1991). Three subspecies are currently recognised: Va.angolensis from Angola and the adjacent part of Zaire, V.a.microstictus from eastern Africa (southern Sudan and Ethiopia south to Zimbabwe and Mozambique) and V.a.albigularis from the remainder of the range. They can be distinguished by the number of scales around the body (75-95 in V.exanthematicus, 122-152 in V.albigularis microstictus, 137-167 in V.a.albigularis and 110-141 in V.a.angolensis (Laurent 1964). V.exanthematicus ionodesi (Laurent 1964) from Tanzania is considered to be a description of juvenile V.albigularis (Strimple 1988/9). V.albigularis is a very variable animal and whilst some people believe that it will eventually prove to be a complex of several species, others find variation between the subspecies to' be too continuous to warrant their recognition. Heck (1955) commented that two specimens caught in the same part of South Africa had very different colouration (slate-grey and red-brown) and Rese (l983b) reports that microstictus and albigularis will interbreed. In the literature V.albigularis is often referred to as V.exanthematicus, creating considerable confusion. An excellent review of this fascinating group of monitor lizards can be found in Strimple (1988/89).
Lengths of up to 213cm have been claimed for the white-throated monitor (Strimple 19811/9), but specimens of I90cm must be rare. The tail is only slightly longer than the head and body length in adults. In many areas they rarely exceed 100-150cm (Stevenson-Hamilton 1947; De WaaI 1978; Auerbach 1985). Mean size of six examined by Bowker (1984) was 41 mm SVL and 2430g. Males may become longer and heavier than females. The largest male recorded in Orange Free State, South Africa was 126cm TL (6lcm SVL) and the largest female was 106cm TL (498cm SVL) (De WaaI 1978). In Etosha National Park, Namibia, largest male was 77cm SVL and 8kg, largest female 62cm SVL and 6.5kg (Alberts 1994). In captivity adults can become extremely obese and exceed 20kg in weight (Loveridge 1943; Branch 1991). The largest specimens may come from Kenya and Uganda. In Namibia females become sexually mature at around 45cm SVL but in South Africa females of 35cm SVL can produce clutches of over ten eggs (Alberts 1994; Branch 1991). Hatchlings measure about 20cm TL and weigh 25- 30g.
White-throated monitors seem equally at home on the ground or in trees. Their dietsconsist largely of invertebrates (especially snails together with beetles, orthopterans, millipedes and scorpions) but they occasionally take larger prey such as other lizards, snakes (including puff adders and spitting cobra), frogs, toads, tortoise, birds, eggs, mammals (including hedgehogs) and carrion (Hewitt 1937; Loveridge 1936, 1942; Fitzsimmons 1943; De Waal 1978; Root 1978; Losos & Greene 1987; Branch 1988, Branch 1991 ; Alberts 1994; Kaufman et al 1994; de Villiers, pers.comm.). It is considered by bird watchers to be more addicted to birds and their eggs than any other Asian or African varanid (Pitman 1962) and this has been substantiated by many direct observations (e.g. Stevenson-Hamilton 1947; Sweeny 1952). Steyn (1992) suggests that goshawks follow monitor lizards in order to feed on animals disturbed by the lizards' foraging activity. Its diet indicates that this is an active forager which eats any suitably-sized animal it encounters. Rose (1962) reports that the lizards will hide in a tree next to a mistletoe bush and ambush birds by stunning them with a blow from the tail . He also reports that a boy who mistook a rock leguaan's head for a stone and tried to throw it at an ox was knocked unconscious by a tail strike. Patterson (1987) suggest that males are territorial and records ritual combat involving opponents intertwining their nodies and attempting to bite each other. Bipedalism does not appear to have been observed in this species although Horn et al (1994) suggest that bipedal combat may occur. Mean active hody temperature of 32.3°C was recorded by Bowker (1984).
This bulky monitor shelters in burrows, rock crevices, abandoned termite mounds or trees. They may be inactive throughout the winter months or during the driest parts of the year and thus build up large fat reserves over the spring and summer. Torpid white-throated monitors have been found sheltering in rock crevices and shallow burrows under rocks during the winter (Bates 1990). Like other African varanids the clutch size is large. Branch (1988) records a female whose 37 eggs accounted for almost half of her weight. In temperate areas eggs are usually laid in the spring and may overwinter and hatch the following spring (Branch 1991). Eggs from female recent imported from Mozambique produced eggs in October (Shaw 1963). Most eggs appear to be deposited in burrows (such as in abandoned nests of ground squirrels - Phillips and Packard 1994) but there are records of them ovipositing in ahandoned termite mounds (Gillet in Branch 1991) and suggestions that they nest in tree hollows (Cowles 1930). Up to 50 eggs may be laid per year (Branch 1991) but in the wild less than half of the eggs are likely to develop and hatch successfully (Phillips & Packard 1994).
A very detailed study of the white-throated monitor in South Africa was carried out by Alfred Brown in the late nineteenth century, but not published until a hundred years later (Branch 1991). This monitor has often been attributed with a nomadic character, wandering over large areas in search of food (Hewitt 1939, Bowker 1984). In Namibia home range size has been estimated at 6.lkm2 for females and 18.3km2 for males (Alberts 1994). Here activity levels are highest during the wet season (January - April) and males are particularly active during the mating period (July-August) when they often walk more than 4km per day. During the dry season they typically move only about 50m per day. This study also demonstrated that males given supplemental food during the dry season are much more active than those who have to fend for themselves. In South Africa the white-throated monitor is most active between October and February (Branch 1991). Densities of whitethroated monitors have been estimated at 10-50 animals per km2 in Kenya and a biomass of around 45,000kg in 22,000km2 at Etosha National Park, Namibia (Western 1974, Phillips 1991)*.
The white-throated monitor has reproduced quite often in captivity and can be extremely prolific with up to 65 eggs laid by a single female in two clutches over six months (Anon 1987; Bom & Bom 1987). At least 10m2 of floor space should be provided (Staedeli 1962; Visser 1981; Anon 1987; Van Duinen 1983; Rese 1983) and under these conditions animals of similar sizes can safely be housed together. They will accept a wide variety of foods including large insects, snails, eggs, small birds, freshwater fish and crustaceans and mammals. Ambient temperatures of 20-35°C are suitable, with hotter basking areas up to 50°C. Cooling the enclosure to 16-18°C for a short period may trigger breeding behaviour when warmer conditions return. Courtship is not particularly violent and ' evidence from captives suggests that females may lay their eggs during the night (Van Duinen 1983; Phillips & Packard 1994). Eggs from healthy females will develop under a variety of incubation conditions (27-31°C) with humidities (measured as water potentials) of 150 --1100K pa and hatch after 116-180 days. Damper conditions produce larger hatchlings whilst different temperatures produce hatchlings with different shaped heads. Data from 78 animals hatched at Iguana Zoo in Vlissingen, Netherlands suggested that warmer incubation temperatures produced females and cooler temperatures produced males (Anon 1987). This has not been substantiated and may be due to difficulties associated with accurately sexing the animals. Hatchlings grow rapidly and may reach sexual maturity within two years. Obviously females require enormous amounts of food if they are to produce their own body weight in eggs each year.
Attribution / Courtesy: Daniel Bennett. 1995. A Little Book of Monitor Lizards. Viper Press U.K.




