Varanus Nilotlcus
Nile monitor, Water leguaan.Varanus niloticus niloticus Linnacus 1758
Varanus niloticus ornatus Daudin 1803
 |
The subspecies V.niloticus ornatus is currently recognised on the basis of having a light coloured tongue and 3-5 rows of light markings over the back, compared to 6-9 rows in the nominate race (Mertens 1942, Loveridge 1953). V.n.ornatus is supposedly restricted to the rainforests and coastal grasslands of western Africa. At present there is no evidence that these animals differ from the nominate race in anything but pattern, although observations on these animals in thick forests are sadly lacking.
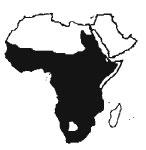
Nile monitors are found almost wherever there are permanent bodies of water. They are absent from deserts but present in most other habitats, from grasslands and desert fringes to rainforests, where they are found along rivers, swamps, pools, lakes and seashores. They will readily inhabit human settlements and cultivations where they are not persecuted. Barbour & Loveridge (l928) record them as high as 2000m above sea level.
In 1929 lngleton published a photograph of a Nile monitor from South Africa which he claimed measured 213cm TL and weighed almost 19kg. He also claimed to have shot another 250cm long. No specimens of this size exist there today but Buffrenil (1992) considered that animals of this size could occur around Lake Chad. Charlton (l973), Patterson (1987) and Fitzsimmons (1934) suggest maximum lengths of about 150cm. Auerbach (l985) gives a figure of over 200cm. Specimens of 188cm (74cm SVL) have been recorded from Orange Free State in South Africa (de Waal 1978) and Boycott & Morgan (l988) refer to a female of 170cm, so it seems likely that in southern Africa occasional specimens may attain lengths of 200cm or more, but they must be rare. A male from Sudan with a SVL of 57.5cm weighed 5.9kg (Hirth & Latif 1979). A healthy male from the coast of Ghana (SVL 49cm) weighed 3,011g and what appeared to be a gravid female from the same location (SVL 44cm) weighed 1,478g (pers.obs.). Around Lake Chad males reach a maximum size of 207cm, females 155cm TL. Typically, males measure 150-17Ocm TL and weigh 5-10kg, females 134cm TL, 3kg. In this area, where the animals are predated upon by man, they attain ages of around eight years (Buffrenil 1992, Buffrenil et al 1994). McGraw (1992) stated that Nile monitors reach 200cm in length around Lake Malawi National Park, and that those from Mumbo and Boadzulu Islands were particularly large. Hatchlings weigh about 30g and measure around 30cm TL.
Changes in skull and tooth morphology with age are well documented in this species (Dumeril & Bibron 1856, Lonnberg 1903, Schmidt 1919, Peyer 1929. Rieppel & Labhardt 1979). Young spedmens have sharp pointed teeth that are gradually replaced with teeth that are broader and develop ridges on the crown. These changes are generally attributed to a shift in diet from fast moving prey such as insect and lizards to a diet of more sessile, but better armoured, prey such as molluscs and crustaceans that must be crushed in the jaws before being swallowed. Where crabs and snails abound they may form a large part of the diet, but in general Nile monitors eat anything they can fit into their mouths. In the Shai Hills of Ghana they appear to be associated with large bat colonies (Bennett & Akonnor, ms). Elsewhere beetles, spiders, orthopterans, snakes, lizards, young crocodiles, fish, small mammals (including domestic cats), birds and their eggs, frogs, toads, crabs, snails, slugs, turtles, termites, caterpillars and reptile eggs (including those of crocodiles, agamids and varanids) are all included in the diet (Hesse 1889; Lonnberg 1903; Roosevelt 1910; Schmidt 1919; Barbour & Loveridge 1928; Cowles 1930; Pitman 1931; Loveridge 1933; Darby 1955; Cott 1960; Cisse 1972; Borquin & Channing 1980; Dial & Vaughan 1987; Patterson 1987; Losos & Greene 1988; Lang & Branch 1990; Van Rhyn 1991; McGraw 1992; Yeboah 1993). They also feed readily on carrion, including the remains of lion kills (Edroma & Ssali 1983), often forage on human waste tips and will even consume human faeces (Bennett & Akonnor ms). Important prey items vary with habitat, but the great variety taken reflects that fact that Nile monitors forage on or below the ground, in trees or in water. Cisse (1972) suggested that they regularly revisit previous excavations to search for food, and may ambush nesting birds. They may use similar strategies to catch migrating shoals of fish (Pienaar 1978). Cott (1960), Modha (1965) and others consider them to be amongst the most important predators of crocodile eggs and young. Ground nesting birds such as water Dikkop often nest in the same areas as crocodiles. It has been suggested that this strategy reduces the danger of other animals, particularly monitor lizards, preying on their eggs (Pitman 1957, Cott 1961). Pitman (1931) suggested that the monitors work in pairs to raid the nests, one diverting the female crocodile's attention and drawing her away from the nest whilst the other rushes in and digs up the eggs. Crocodiles will eat Nile monitors when they can catch them. Other known predators include mongooses and cobras (Branch & Branch 1992; Hinkel 1987).
Nile monitors are most often seen basking on rocks and branches or in the water. Adults can easily outrun people over short distances, even over open ground and will almost invariably make for water when pursued. They retreat to burrows and abandoned termite mounds at night, but in warm weather they may remain outside, sleeping on branches or half submerged in water. Rose's (1962) report that they line their burrows with cow dung is unsubstantiated. Cisse (1972) found that in Senegal the animals left their burrows in the morning and did not return until late afternoon. This is confirmed by Edroma & Ssali (1983) who also note that whereas in warm weather rock or sand are the usual basking substrates, in cooler weather grass and branches are preferred. Of 35 burrows examined by them, none had been dug by the lizards themselves.
During the dry season in tropical Africa and in the cooler months in temperate regions activity is reduced or suspended (Cowles 1930, Cisse 1971). Juveniles are better at climbing than adults, but even large Nile monitors will climb readily. However they lack the dexterity to be classed as truly arboreal: Loveridge (1923) reports that specimens sleeping in trees have been known to loose their grip and plummet to their deaths. They are, of course, superb swimmers. In the wild Nile monitors can remain underwater for more than an hour (Ingleton 1929). Experiments with small specimens (1.4-4.8kg) in captivity have shown that most dives last for 12-15 minutes, never more than half an hour. During these dives heart rate and blood pressure drop (Wood & Johansen 1974). Hinh & Latif (1984) recorded an average body temperature of 28.8°C, with a range from 20-37.2°C with activity above 26°C. An active specimen caught in the late afternoon had a cloacal temperature of 32°C whilst ambient temperature wus 25.3°C (pers. obs.)
Population densities of 40-60 Nile monitors per km2 have been recorded in northern Kenya (Western 1974). Our preliminary studies suggest that populations are equally high in parts of Ghana, where this species is completely protected. Very high densities are recorded for heavily exploited populations around Lake Chad (Buffrenil 1992). However despite its abundance, size and economic importance the Nile monitor is a very poorly studied animal. The most complete published· study of their ecology is from Natal, South Africa (Cowles 1928. 1930). Here they were found close to water between September and March and were relatively inactive during the rest of the year, sheltering in abandoned termite mounds. Cowles was also the ftrst person to note the use of active termite mounds as nest sites. The nests used are large mounds, usually situated close to water. The female tears open the nest and deposits her eggs without attempting to cover them. The tennites quickly repair the nest and the eggs are incubated safely, with constant heat and humidity, providing the mound remains occupied by termites. Cowles (1930) suggested an incubation period of about 300 days, based on his observations of hatchlings emerging in November and December and estimated that eggs were laid during the hottest part of the year (December to February). However Branch & Erasmus (1982) record a female in Transvaal seen depositing her eggs in August (at the end of the winter) and considering known incubation times in captivity (see below) it seems likely that the duration of incubation is less than half, or even a third, of that suggested by Cowles. In more northern parts of Africa suitable termitaria appear to be unavailable and eggs are deposited in burrows. In Sudan eggs may be laid burrows dug into the clay walls of irrigation canals (Clouds ley-Thompson 1967). In Senegal eggs are laid from late October to late December during the wet season. Gravid females have been found in Tanganyika during November (Barbour & Loveridge 1928) and during early July in Zanzibar (Pakenham 1983). In Ghana we have found what appeared to be gravid females in August and September.
Large female Nile monitors can lay enormous clutches of eggs. According to Loveridge (1934) up to 60 eggs are produced in a single clutch, Borquin (1991) found 63 eggs in a termite mound and there are many records of clutches containing over 35 eggs (e.g. Barbour & Loveridge 1928, Pakenham 1983, Boycott & Morgan 1988). According to Buffrenil (1992) females mature at around 120cm TL., around Lake Chad and initially produce only about ten eggs per year. Like other African monitor lizards, the Nile monitor enhances its chances of survival by producing large numbers of eggs that hatch comparatively quickly. Most eggs weigh 46-52g and measure about 6×4cm. Therefore the weight of a clutch of eggs from a large female can approach 3,000g! It appears that females spend most of the spring and summer feeding heavily and accumulating massive fat reserves which are then converted into egg yolk in the liver during the long period of inactivity during the winter. In less temperate parts of Africa the period of activity occurs during the dry season and may be shorter in duration (Buffrenil 1992). This is reflected by the apparent smaller clutches of females from West Africa (e.g. Cisse 1976). The need for females to accumulate large fat deposits and then greatly reduce activity during ovagenesis appears to be important in a number of both temperate and tropical varanids.
Surprisingly there are few published accounts of interactive behaviours such as ritual combat or courtship in Nile monitors. This can be attributed to the secretive behaviour of these huge lizards. Adults have been seen wrestling on the ground (presumed to be courtship by Clements (1968) but interpreted as ritual combat by Horn (1985). They have also been observed standing on their hind legs (Wearne 1962). but bipedal combat has not been observed to occur in this species. The Nile monitor makes great use of its tail for defence and the battered condition of these appendages in old specimens is attributed to its regular use as a club with which to deter aggressors. A number of unusual behaviours have been noted amongst Nile monitors. A young monitor lizard that fell into a enclosure full of young (30cm) crocodiles seized several of the crocodiles and turned them onto their backs before being removed. The crocodiles were estimated to weigh twice as much as the lizard (Pooley 1968). According to Stevenson-Ham,lton (1947) a monitor surrounded by four large lion cubs kept perfectly motionless apart from occasionally twitching its tail tip. The lion cubs watched closely, but appeared to interpret the movements as that of a snake and eventually wandered away. The same author reports that an eagle which seized a Nile monitor was in turn seized on the thigh by the lizard, which steadfastly refused to let go. When found by a ranger the bird was in a state of utter exhaustion.
There are few lizards less suited to life in captivity than the Nile monitor. Buffrenil (1992) considered that, when fighting for its life, a Nile monitor was a more dangerous adversary than a crocodile of a similar size. Their care presents particular problems on account of the lizards' enormous size and lively dispositions. Very few of the people who buy brightly coloured baby Nile monitors can be aware that, within a couple of years, their purchase will have turned into an enormous, ferocious camivore, quite capable of breaking the family eat's neck with a single snap and swallowing it whole. An enormous enclosure (at least 8m2 and 2.5m high) is needed to keep these animals properly, furnished with a sizeable pool of water and plenty of tree trunks for the lizards to climb on. A period of inactivity during the cool winter or hot dry season (depending on the origins of the animals) is desirable. Nile monitors are prone to obesity and the diet should include significant quantities of non-mammalian food. A temperature range of 8l-30°C is suitable with basking spots up to 45°C. Very few records of captive breeding are available (Enright 1989). Animals should be housed apart except at breeding time. The small size of females suggests that finding a compatible pair could be particularly problematic. Best results would be obtained by obtaining a group of young, unrelated, animals and rearing them to adulthood. Eggs collected in the wild or laid by wild caught females hatch after 141-150 days at 27-30.5°C, 120-137 days at 30°C and 92 days at 32°C (Boycott & Morgan 1988, Branch & Erasmus 1982, Olmstead 1987, Enright 1989). In captivity they are known to practise cannibalism (Charlton 1973). Animal dealers who sell Nile monitors under the pretence that they can be kept as pets are a despicable breed, but given spacious surroundings and suitable furnishings Nile monitors can be very rewarding animals to keep in captivity.
Nile monitors are eaten in many areas and their organs and tissues used for medicinal purposes (Anon 1937). The skin is very durable and beautiful. Between 1980 and 1985 trade in live specimens averaged 816 specimens per year whilst trade in skins averaged over 40,000 per year. In 1988 more than 700,000 skins were exported (Luxmoore & Groombridge 1990; Buffrenil 1992). Most skins are exported from Mali, Nigeria, Cameroon and Sudan to Europe, particularly France. The uses of monitor lizards and the effects of exploitation for meat and skins around Lake Chad is documented by Buffrenil (1992, 1993, et al 1994). In Ghana the Nile monitor is known as mampan tintin, in Zambia as mbutu, nabulwe, hopani, nsamba or imbulu (Broadley 1971). An extensive list of the common names of African monitor lizards can be found in Auerbach (1995).
Attribution / Courtesy: Daniel Bennett. 1995. A Little Book of Monitor Lizards. Viper Press U.K.




