Varanus Flavirufus
 |
Desert sand goanna
Varanus flavirufus ssp.
Sand goanna, goanna x
Varanus gouldii was frr st described by Grey in 1838. Subsequently the animals from the extreme south of Australia were classified as a separate species (V.rosenbergi) and a desert race was described (V.gouldii flavirufus) . In 198O Storr desaibed a new species V.panoptes, from animals previously assigned to V.gouldii, based on differences in scalation, the presence of rows of dark spots over the back in the V.panoptes rubidus and the presence of a banded tail tip in V.panoptes panoptes. Unfortunately the type specimen of V.gouldii is kept on the other side of the world (in London) and Storr did not see this animal before describing V.panoptes . In 1991 Bohme reported that the type specimen of V.gouldii was identical to the animals described by Storr as a new species. As a result V.panoptles is considered a junior synonym of V.gouldii and so the "new" species is entitled to the old name V.gouldii. Meanwhile the animals considered by Storr to belong to V.gouldii have no valid scientific name. The next available name is V.f1avirufus, the name used by Mertens (1958) to describe the desert races of "V.gouldii". Thus the desert form becomes V .f1avirufus flavirufus, but the remaining races, which extend throughout Australia except for the extreme south, are currently nameless. This is highly unsatisfactory, because some people believe that the desert populations (V.f1avirufus) form a separate species from the animals in more mesic areas, and that the latter animals (which now have no valid scientific name) may be a complex of more than one species. This makes any description of the group ridiculously complicated. Biochemical comparisons of the group throughout Australia are needed to properly resolve these very serious taxonomic problems. When adequate material is available to allow comparison between races from all over Australia any revisions to the taxonomy will have to allocate new names to animals that have been written about for over 150 years.
In summary; the animals referred to here as sand goannas or goanna x (V.flavirufus) are usually called V.gouldii gouldii in the literature. The desert sand goanna V.f1avirulus flavirufus is usually called V.gouldii flavirufus. The animals known as V.panoptes in the literature should be called V.gouldii, and the animals known as V.gouldii in the literature actually belong to V.flavirufus. In older literature the name V.gouldii could describe the nameless actuality, V.gouldii gouldii, V.g.rubidus, V.g.horni, V flavirufus or V.rosenbergi. Often it is impossible to deduce the correct identity of the animals concerned. Here I have tried to omit such information.
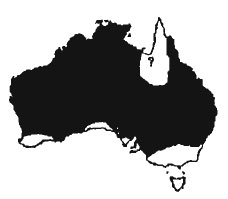
V.f1avirufus is a very widespread species. They are found throughout Australia except for the extreme south (where they are replaced by V.rosenbergi) the extreme south-east (where goannas appear to be entirely absent) and parts of central Western Australia and northern parts of Western Australia and Northern Territory (where they are replaced by Gould's goannas). The taxonomic status of the animals on the islands off the northern coast of Australia is not clear, but considering that Gould's goannas are common both on the northern coast and in southern New Guinea islands such as Groote Eylandt in the Gulf of Carpenteria are quite likely 10 belong to that species. Sand goannas are rarely sympatric with Gould's goannas. The two species appear to have different habitat requirements. The sand goanna is restricted to sandy soils whilst Gould's goanna prefers harder substrates. In general it can be said that they will inhabit any area with sandy ground, including forests, woodlands and scrublands. They are common in many areas (e.g. Onslow region (Storr & Hanlon 1985b), Bundaberg Region (Richardson 1976), Karrakatta Cemetery, Perth (Thompson 1992)) but reported to be uncommon around Shark Bay (Storr & Harold 1978), on the Nullabor Plain, Western (Brooker & Wombey 1978) and in Merriwindi Forest (Bustard 1968). Additional location data can be found in Mertens 1958; Storr 1980; Morris & Rice 1981; Mcllroy et al 1985: Grundke & Grundke 1992b; Shea 1994).
Pattern and colour vary throughout the range. Storr (1980) suggested that specimens from the Kimberleys, which are darker and have less pattern than those found elsewhere, may constitute a separate race. The desert race V. flavirufus flavirufus has a brighter pattern and more reddish colouration than the anonymous form. Intermediate specimens are recorded from the Carnarvon Range and other desert fringes. Schmida (1985) notes that in the Sydney sandstone region Gould's goannas have a banded tail tip and are black with rows of prominent yellow spots. The banded tail tip is generally taken as being characteristic of Gould's goannas rather than sand goannas, which invariably has an unbanded tail tip.
Around Jabiru in tropical Northern Territory the sand goanna reaches a length of at least 42.5cm (males) and 33cm (females) SVL and becomes sexually mature around 32cm (males) and 28cm (females) SVL (Shine 1986). In the same area Gould's goannas grow to at least 67cm SVL and matures at 39cm (males) and 3lcm (females) SVL. In the Barky Tablelands of Northern Territory sand goannas grow to at least 40cm SVL (Pengilly 1981). On Archer River in the Cape York Peninsula, Queensland they reach a total length of about 90cm (Glazebrook 1977). Three specimens collected in south-eastern Queensland weighed 10732562g (the largest had a total length of 9lcm) (Johnson 1972, 1976) and specimens collected near Brisbane weighed between 89-1020g (Bartholomew & Tucker 1964). Largest found in a cemetery at Perth by Thompson (1992) was 1330g. Specimens of 30-35cm SVL weighed 300-750g (Thompson 1994). According to Storr (1980) the sand goanna reaches a total length of 100cm in Western Australia. Thus there appears to be considerable variation in the maximum size attained by these monitors in different areas.
Although they will climb trees to escape from predators, this is primarily a ground dwelling lizard. In the labiru area the sand goanna is found only in sandy woodlands whereas Gould's goanna inhabits woodlands, grasslands and is most common around rivers and pools. The sand goanna feeds mainly on mammals (bandicoots, mice and rodent-like marsupials) and other reptiles (skinks, geckoes, agamids, frogs, monitor lizards, snakes and reptile eggs). They also feed on insects (particularly lepidopteran larvae, orthopterans and beetles), fish and Irustaceans (Shine 1986). Around the McKinley River in Queensland sand goannas are important predators of freshwater crocodile eggs (Crocodylus Johnstoni) destroying up to 55% of their nests (Webb 1982). In Perth the diet of the sand goanna consists mainly of large orthopterans and spiders (Thompson in Pianka 1994). Elsewhere sand goannas are recorded to prey on birds and their eggs, tiger snakes. rabbits. frogs. scorpions, centipedes, wasps and ants, (Barrett 1928; Fleay 1950; White 1952; Berney 1936; Bustard 1970; Pengilley 1981; Losos & Green 1988). Stammer (1981) records that specimen that mouthed a marine toad (Bufo marinus) died shortly afterwards.
The sand monitor is an excellent digger. Its limbs are comparatively longer than those of other varanids and its feet are huge and equipped with powerful claws. Glazebrook (1977) records that a specimen excavated a hole 76cm deep in 20 minutes. It probably retrieves much of its food from below the ground, suggesting that olfaction is at least as important as sight in prey detection. They have been seen striking at prey animals hiding under stones with the tail, in attempts to move them to more accessible spots (Eidenmuller, pers. comm.). Bustard (1970) found them particularly associated with rabbit warrens. He uses the name racehorse goanna (by which V.tristis is also sometimes known) in reference to the speed at which they move over flat ground, and describes their ability to run bipedally for short periods. Pianka (1970) notes that when walking normally desert specimens they do not drag their tails. However bulkier animals from wetter habitats appear to leave a clear tail trail (SprackJand 1992). Ritual combat is described by Thompson et al (1992).
In tropical areas eggs are laid in the wet season (January and February), in temperate areas during the spring and early summer (Shine 1986; Thompson 1994). Clutches comprise of between 3-11 eggs, measuring an average of about 6×3cm (Shine 1986). Berney (1936) records a specimen that laid five eggs in October (presumaably in southern Queensland). He believed that the lizard might have picked up three eggs in its mouth and carried them to another hole when it was disturbed. A 60cm TL female laid eight eggs in captivity (Barnett 1979), another laid seven eggs (Irwin 1986). The sand monitor may oviposit in active or abandoned termite mounds when these are available (Cogger 1967; Bustard 1970; Green & King 1993). In temperate areas the Lizards' activity is greatly reduced during the coldest months and even during the warmer months activity may be restricted to early morning and late afternoon (Green & King 1978). In tropical northern Australia sand goannas may have a similarly restricted activity pattern, with most movement during the mornings (Shine 1986). Ln South Australia sand goannas are most active during the swnrner. Daily activity ranges vary between 6,100 and 32,500m2 depending on season (Green & King 1978). At a cemetery in Perth Thompson (1992) estimated foraging areas over 4-18 days to be between 841 and 13,100m2. In this habitat they moved to new areas every few days. Where leaf litter (which contained most of their prey) was abundant the Lizards tended to forage in smaller areas. During the breeding season activity area averaged 90,000m2 with the larger animals covering greater areas (Thompson 1994). In the Northern Territory Pengilley (1981) collected road killed specimens and found a bias of 4: 1 in favour of males.
Active body temperatures of sand goannas measured in the wild average 37.7°C in the Great Victoria Desert, with body temperatures as high as 44.7°C recorded (Pianka 1994). Mean body temperatures of 34.4-36.2°C are recorded by Light et al (1966). Telemetric studies have shown a range of 27.2-38.1°C (King 1980). In laboratory studies Johnson (1972) found that animals from central 'Queensland tolerated body temperatures of up to 40°C (head temperatures of 37.6°C) without signs of distress.
The desert sand goanna (V.flavirufus flavirufus) is found in desert regions of Western Australia, Northern Territory and South Australia. Storr (1980) considered the variation between this race and the nameless variety to be too gradual to warrant different names. However the limited information available suggests that the desert form leads a very different life to its counterpart in wetter areas.
Desert sand goannas rarely exceed 100cm in total length. None of the specimens I have seen possess the bulk of adult V.rosenbergi or the nameless race. The largest specimen found by Thompson & Hosmer (1963) in the Great Sandy Desert measured 76.4cm TL. Those found in the Great Victoria desert have an average SVL of 32cm. with sexual maturity attained at around 25cm SVL. Males have significantly larger heads than females and may reach slightly greater lengths, (Pianka 1994). A healthy specimen at Laverton. Western Australia, with a SVL of 31cm (75cm TL) weighed 380g during midsummer (pers. obs.).
In the southern deserts atleast activity is restricted to the warmer months (September to February). The remainder of the year is spent underground. V.flavirufus flavirufus (and probably other closely related races) often shelter in shallow burrows that terminate just below the surface, allowing the animals to beak through the sand and escape if persued into the burrow (Bustard 1970; Greer 1989). Breeding in the Great Victoria Desert occurs from September to November. Clutch size may be smaller than in the nominate race; Pianka (1994) records a maximum of eight eggs in the Great Victoria Desert, Brooker & Wombey (1978) record seven eggs from a female in the western Nullarbor Plain. Bredl (in Bustard 1970) cites the case of a 107cm TL female from South Australia that laid 11 eggs in captivity. Hatchlings appear in January and February in many areas of Western Australia (Pianka 1970). Given the known incubation times in captivity it seems likely that the eggs take about a year to hatch in the wild. Sand goannas grow very quickly. Pianka (1994) suggests that they reach sexual maturity within a year. A specimen kept at the Dallas Zoo grew from 30cm to 75cm in 19 months (Murphy 1972). Irwin (1986) records that 27cm TL hatchlings grew to 33cm in three months. Eggs laid in captivity weigh 15-18g (Mitchell 1990) and in the wild desert sand goannas weigh around 11-15g shortly after hatching (Pianka pers. comm.). Card (1994b) records that hatchling sand goannas can weigh as much as 38g.
Mammals do not appear to be as important a food for the desert race as for those from wetter regions. In the desert lizards (agamids, skinks, geckoes and varanids) and reptile eggs are their most important prey. Pianka (1994) list 27 species of lizards preyed upon in the Great Victoria Desert. They also eat large numbers of beetles, orthopterans, spiders and centipedes as well as other small invertebrates, mammals and nestling birds. They will feed on carrion when the opportunity arises and take poisoned bait intended for introduced pests. Juvenile are more insectivorous, feeding especially on roaches (Pianka 1970, 1994). Houston (1978) reports that the desert goanna will frequent temporary pools in the desert during the rains and are able to remain underwater for at least several minutes. The habit of standing bipedally is well documented for Gould's goanna, but my impression is that V.flavirufus .flavirufus is less inclined to adopt a bipedal stance than Gould's goanna or goanna x. probably on account of its smaller body size.
Sand goannas have been bred in captivity a number of times (Barnett 1979; Irwin 1986; Mitchell 1990; Card 1994a&b, 1995b). Care must be taken to ensure that both members of a prospective pair belong to the same race. There appear to be no obviously external differences between the sexes, other than the larger head size of males referred to above. A number of authors testify to the aggressive nature of this species in captivity (e.g. Longley 1947; Johnson 1976; Delean 1981). In small enclosures larger specimens will eventually attack and injure any smaller room mates. Animals of similar sizes should be introduced to each other in spring and the male removed as soon as copulatory behaviour ceases. In large enclosures groups of animals can be kept together safely. Irwin (1986) kept five adults together in an enclosure with 78m2 of floor space. They do well on a diet of small lizards, mammals and birds supplemented with large insects, vitamins and minerals. In captivity several clutches of eggs may be laid in a year, which hatch after 169- 265 days. at 29-32°C. Youngsters will immediately accept lizards and small mammals as well as insects. They grow rapidly and should be housed separately. Garret & Card (1993) record that newly-hatched individuals respond more to the smell of crickets than to the smell of mice and geckoes. A specimen at the Dallas Zoo survived for over 18 years in captivity (Snider & Bowler 1992).
Attribution / Courtesy: Daniel Bennett. 1995. A Little Book of Monitor Lizards. Viper Press U.K.





