Varanus Griseus
Desert monitor, grey monitorVaranus griseus griseus Daudin 1803 Grey monitor
Varanus griseus caspius Eichwald 1831 Caspian monitor
Varanus griseus koniecznyi Mertens 1942 Indian desert monitor
 |
Mertens (1954) distinguished between the subspecies of V.griseus largely on the basis of pattern;
V.griseus griseus has 5-8 narrow grey bands on the back and 19-28 bands on the tail which extend almost to the tip. The tail is round in cross section, 131·146% of SVL.
V.griseus caspius has 5-8 bands on the back and 13-19 bands on the tail with a plain tail tip. The tail shows significant lateral compression and is 118-127% of SVL. There are about 143 rows of scales at midbody,
V.grseus koniecznyi has 3-·5 bands on the back and 8-15 bands on the tail with a plain tail tip. Tail may show some lateral compression, but not to the elltent seen in V.griseus caspius. It is 118-I27% of SVL. In addition its head is broader and flatter than that of V.griseus griseus. There are 108-139 rows of scales at midbody.
His descriptions were based on a very small number of specimens and it is likely that this taxonomy will be revised at some time in the future, hopefully using biochemical features rather than external ones.
The desert monitor is an magnificent animal. It is my favourite monitor lizard for several reasons. It inhabits some of the most hostile regions of Earth, experiencing blistering heat in the summer and extreme cold in the winter. Grey monitors are lizards with attitudes. They are very spirited creatures which cannot be tamed and they appear to hate hunanity wih a vengeance. In turn, people generally hate the grey monitor and it is persecuted almost everywhere it is found. The energy and spirit of its threat display are doubtless responsible for the many sinister characters that are attributed to it by humanity. They are believed by many to be as venomous as any snake, and are also supposed to be capable of inflicting a variety of deforming, debilitating and terminal illnesses on any unfortunate person they encounter. In fact the grey monitor is probably a benefit to humanity where it is allowed to survive unmolested. They eat large numbers of crop-destroying beetles and larger specimens consume adult cobras and vipers with great relish. Its defensive posture is largely bluff and is only assumed if the animaI is cornered and unable to escape. In captivity the desert monitor is not particularly co-operative. They have been kept in captivity for over a century (Von Ralhgen 1894), but only a single captive breeding has been reliably reported. Our failure with the captive maintenance of this species is largely attributable to a lack of understanding about its way of life in lhe wild and in particular about its seasonal behaviour.
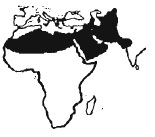
The grey monitor V.griseus griseus occurs in lhe deserts of north Africa (from Morocco and Mauritania east to Egypt and Sudan),the Arabian Peninsula (although it appears to be absent from the island of Bahrain). south-eastern Turkey, Syria, Israel, Palestine, Lebanon, Jordan and Iraq (e.g. Haas 1951; Hass & Battersby 1959; Anderson 1969; Schmidt & Marx 1956, 1957; Schmidt 1919; Aktan 1971, Arnold 1984; Bishai 1960, Martens & Kock 1992). It reaches a maximum size of about 120cm TL in Iraq (Khalaf 1959) and 100cm TL in Israel, but in Algeria specimens of 80cm TL are rare (Mammeir. pers. comm.). Tolal size anained seems to depend on the climate of the area, with largest specimens coming from areas which yield more food or allow longer activity seasons. Mosauer (1934) noted that specimens from Tozeur and Nefta in Tunisia were smaller than those from Gafsa. The largest male found during a comprehensive study in the coastal plains of Israel was 46cm SVL and 1,261g, the largest female 38cm SVL and 702g (Slammer & Mendlessohn 1987). In captivity however they can attain weights in excess of 3,000g. The tail varies between 120-150% of SVL. Details of scalation and morphology can be found in Mertens (1954). The pattern and colouration of this lizard shows great variability. In dry areas with little vegetation (e.g. the sand dunes of Mauritania) grey monitors may have no pattern at all, in wetter areas with more luxuriant plant growlh the animals can have very bright patterns. Juveniles are always brighter than their parents.
In Israel grey monitors tend to avoid beaches and keep to more inland areas. They inhabit sand dunes and are not adverse to areas of human activity including rubbish dumps and building sites (Stammer & Mendlessohn 1983, 1987). Anderson (1963) records lhem from as high as 1,200m above sea level and Arnold (1984) records them from a variety of substrates induding gravel in Arabia. In the Sahara desert lher inhabitual regions but are much more common in lhe more humid zones (about 6 per km2) lhan in more arid areas (about 2 per km2) (Vernet 1982). In lhe coastal plains of Israel Stanner & Mendlessohn found about 4 specimens per km2. Perry & Dmi'el (1995) note that the grey monitor has disappeared from many disturbed dune areas.
Not surpisingly the diet of the grey monitor varies enormously depending on its habitat. Where other vertebrates are plentiful they tend to form the bulk of their prey. In Israel other lizards and snakes are their most common prey, along with ground-nesting birds, tortoise and the eggs of all these animals. They also prey on toads and mammals (including gerbils and young hares) and will take carrion including dead hedgehogs and cats (Stanner & Mendlessohn 1986). Invertebrate prey are less important than in many other desert-dwelling monitor lizurds. but include beetles. orthopterans. heteropteran bugs. ants. snails. centipedes and scorpions. This wide variety of prey suggest that like many monitor lizards. the grey monitor devours any animal of a suitable size that it encounters. Diet in Algeria appears to be very similar (Vernet & Grenot 1973). Seven stomachs of animals from a variety of locations examined by Losos & Greene (1988) also contained lizards and snakes. Aktan (1971) found two brge lizards and pieces of eggshell in a specimen from Turkey. Lizards. snakes and mammals have also been recorded from the stomachs of animals in Arabia (Arnold 1984. Tilbury 1988). Egypt (Schmidt & Marx 1957. 1958) and Tunisia (Mosauer 1934). Andres (1904) rel:ords that adults will eat small puppies.
The deserts inhabited by the grey monitor are subject to great variations in seasonal temperature. In Israel males are active from late April and early May until July or August whilst females remain active until late October or November (Stanner & Mendlessohn 1983. 1987. 1991). The lizards stick to well defined home ranges and those of males are much larger than those of females (about 1km2 compared with 0.3km2). Females maintain the same home ranges for at least several years whilst males change theirs annually. Foraging distances of 2km or more are common
In Algeria the monitors have two periods of activity, from April to June and a shorter period during October. During the winter and hottest part of the summer they remain below ground. Horne ranges were estimated at 1-4km2 (much larger than those in Israel, presumably because of lower food densities) and foraging trips of up to 8km per day were recorded (Vernet 1982: Vernet et al 1988a). These studies suggested that males were not particularly more active than females. In the same parts of Algeria Saint Girons & Saint Girons (1959) estimated home range size as 2-5km2. Although the lizards have well defined home ranges they are neither territorial nor do they consistently use the same shelters and basking sites. In Syria grey monitors are found only in spring and early summer (Martens & Kock 1992) whilst in Mauritania active grey monitors have been seen as late as November (Linley. pers. comm.).
In Algeria grey monitors maintain active body temperatures of between 35-38°C. At temperatures below 20°C the lizards become inactive and hibernation is induced at about 17°C. The lizards voluntarily allow body temperature to get no higher than 41°C and die at temperatures of between 44-47°C (Vernet et at 1988b). Grenot (1968) (reviewed in George ( 1986)) found that the grey monitor is able to maintain a body temperature of 42°C for up to four hours at an ambient temperature of 50°C. In warm weather the lizards can raise their body temperatures by as much as 0.50°C per minute by basking (Francaz et al 1976, 1978). Although the seasonal activity of the grey monitor depends largely on temperature, Vernet et al (1988) believed that the daily activity of the lizards depended more on prey availability than climate (similar findings have been reported for V.arbigularis). Lemire & Vernet (1985) report that the grey monitor has salt glands which allow it to minimise water loss.
In Israel grey monitor burrows are said to be an average of 125cm long and just 30cm deep. They vary greatly in shape (Stanner 1985). These burrows are probably used only during the wanner part of the year. During the winter the lizards may take shelter in much deeper refuges. An Algeria they have been recorded from burrows as deep as 300cm (Marnmeir.pers. comm.). Schmidt & Marx (1957) record that in Egypt grey monitors dig very deep and complex burrows with several openings. Andres (1904) records that a captive specimen escaped by digging a very deep burrow under a garden wall.
Little is known about the breeding behaviour of the grey monitor lizard. In Algeria, Tunisia and Israel mating occurs during May and June and eggs are laid in June and July (Vernet, Lemire & Grenot 1983; Mertens 1942; Stanner 1985). There are no records of nesting sites. In Israel females' weight may be reduced by as much as 47% after egg laying. Their continued activity into the early winter is probably due to their need to accumulate large fat reserves both to maintain them through the long period of inactivity and to provide energy to fonn eggs for the next breeding season. Hatchlings appear in March of the following year, but an incubation period of eight months seems unlikely (see below). The habits of the juveniles remain completely unknown. Thilenius (1897) suggests that females may return to the nest site after oviposition, but makes no attempt to explain why.
The Caspian monitor (V.griseus caspius) is the largest race of the desert monitor. It is considered to be in danger of extinction and is one of the few monitor lizards whose commercial trade is completely outlawed. Despite this they are regularly offered for sale and, a few years ago at least, were not an uncommon sight in Moscow's pet market. The Caspian monitor is found from the east coast of the Caspian Sea throughout the deserts of central Asia (including many islands of the Aral Sea as far as about 690 east and 460 north. In the south it is found at elevations of up to 800m in the Kopet Dag mountains and extends into northern Iran, western and southern Afghanistan as far as western Pakistan. It is best known from the states fonneriy affiliated to the U.S.S.R.; Turkmenistan, Khazakstan, Uzebekistan, Kirgizstan and Tadzikistan. It previously occurred in the Fergana Baisin but is now extinct there and has also disappeared from the Golodnaya and Dalverzinskaya regions of Uzebekistan (Leviton & Anderson 1970; Yadgarov 1968, el al 1988; Ataev 1987; Shammakov 1981; Makayev 1982). Specimens have also been recorded from as far east as Tasihkent (Kulagin in Nikolskii (1915)) but they do not occur there now.
The Caspian monitor reaches a maximum size of about 140cm. Males tend to be longer than females (largest male recorded from Turkmenistan by Shammakov (1981) was 58.5cm SVL, largest female was 46cm) but not much heavier (heaviest male was 2,850g heaviest female 2,700g). The Caspian monitor is distinguished from other races of V.griseus largely by the shape of its tail, which is laterally compressed in contrast to the tails of other races, which tend to be more or less rounded in cross section.
The Caspian monitor is found in both sandy and clay deserts. They avoid areas of dense vegetation but are found in sparse woodland. Those from clayey areas are often a distinctive reddish colour (Bennett 1992a). On Islands in the Aral Sea they are found in salt marshes as well as sandy areas. They are sometimes found on the edges of agricultural land, but, because they are often killed when encountered, Caspian monitors tend to be very uncommon in areas of human habitation. In abandoned settlements they often inhabit cracks in wattle and daub houses. Caspian monitor lizards reach their highest densities where colonies of mammals are abundant. Makeyev (1982) records 9-12 specimens per km2 on the edge of Karabil, close to Karamet-Niyaz (Turkmenistan) and in the Saihan Valley (Uzbekistan), 5 specimens per km2 in southeastern Turkmenistan and 3-5 specimens per km2 on clay desert at Kara-Kala. Over most of the sandy desert densities are estimated at 2-3 lizards per km2, whilst in river valleys numbers drop 10 1-1.5 specimens per km2
The diet of the Caspian monitor is similar to that of the nominate race. In many areas they feed on hatchling tortoises which emerge in the spring and excavate and consume tortoise eggs which are laid in May. They also eat small mammals. including the giant gerbil and young hares. In Turkmenistan adults prey extensively on large snakes, cobras and vipers up to l40cm are swallowed whole. Shammakov (1981) lists 10 species of lizards, six snakes, four birds and six manunals that are regularly eaten by the monitor lizards. In Kyzulkhum their diet consists mainly of rodents (especially the giant gerbil) and far fewer numbers of lizards and invertebrates than are consumed in the Karakhum. In the former area the lizards regularly enter burrows in search of mammalian prey, whilst in the latter region they tend to forage mainly on the surface (TseUarius et al 1991). In the Surhandarja Baisin they feed largely on young tortoise, small mammals and invertebrates (especially tenebrionid beetles) (Yadgarov 1968). Caspian monitors will also prey on srnaller members of their own species (Makarov 1985). The lizards ability to prey on snakes that are extremely dangerous to man is particularly interesting. Experiments to detennine the Caspian monitors' ability to tolerate viper and cobra venom by injecting them with venom sufficient to kill up to 4,000 adults humans seem to indicate that the lizards have considerable resistance to both haemotoxic and neurotoxic venoms (Rjumin 1968).
In Turkmenistan Caspian monitors commence activity in late March and early April. Like other desert monitors they are active during the middle of the day in spring, but as temperatures rise they adopt a bimodal activity pattern, foraging early in the morning and late in the afternoon but spending the hottest part of the day below ground. By September and October temperatures are cool enough for them to resume a single period of activity, but they commence hibernation earlier than most other lizards and have virtually disappeared by early October (Shammakov 1981). Activity temperatures of 31.7 -40.6°C have been recorded (Sokolov et at 1975; Tsellarius et al 1991). The Caspian monitor is capable of running at speeds of up to 20km per hour over short (100-150m) distances. Home ranges of over 1km2 have been detennined for adults (Tsellarius et al 1991). The same study suggested that Caspian monitors may mark their territory in spring and early summer, in contrast to the work of Stanner in Israel which found no evidence of scent marking·. Foraging trips may ex tend to over 10km per day (TseUarius & Cherlin 1991).
Caspian monitors are strong diggers. In clay desert where the substrate is too hard to excavate they shelter in mammal burrows and in river Valleys they often utilise the burrows of ground dwelling birds. In sandy desert burrows are often more than 500cm long and typically 50-120cm deep. Burrows used during the spring and summer tend to be in more open areas than those used for hibernation, which are dug under bushes Yadgarov 1968; Makayev 1982; Bennett 1992b). Mating occurs during April and May. Up to 34 eggs are laid in a burrow 70-114cm deep usually situated on a slope during June and July. Sometimes the burrows of rodents are used as nesting sites. Females spend up to a week digging test holes and preparing nests, guard the eggs for several weeks after egglaying and have been reported to return to the vicinity of their eggs around hatching time. The eggs hatch in September or October but the youngsters remain together in the nest and do not commence activity until the following spring. Eggs laid in captivity weigh 32-35g (Yadgarov 1968; Shammakov 1981; Tsellarius & Menshikov 1995; Dujsebayeva 1995; Makayev. pers.comm; Kudryatsev pers. comm.). Sexual maturity is attained within three years (Shammakov 1981). Tsselarius and Cherlin (1991) note that many Caspian monitors have scars on the back, which they attribute to attempted rredation by birds, but are interpreted in other species as wounds recieved during ritual combat. Bipedal combat occurs in the typical fashion of large monitor lizards (Tsellarius 1914).
Koniecznyi's monitor. or the Indian desert monitor is found in central and western Pakistan and India. Auffenberg et al (1989) provide extensive distribution data. They considered that the subspecies Occurs only in the past and present Indus valley and is largely restricted to sandy desert , being uncommon in day deserts. Auffenberg (in Luxmoore & Groombridge 1990 suggests a density of 9 lizards per km2 in the desert scrub of Pakistan. Sprackland's (1992) claim that their range extends "nearly to Burma" is unsubstantiated and seems unlikely considering that this species is restricted to desert areas.
Koniecznyi's monitor is the smallest subspecies of V.griseus . Males reach a maximum length of 84cm TL (37cm SVL) and a weight of 580g. Females grow to 75cm TL (34cm SVL) and weigh up to 520g. Sexual maturity, for females at least, is attained at about 24cm SVL. They reach maximum weights in September and October (Auffenberg et al 1989). These lizards are inactive between December and March, or at least they stop feeding. Corkill (1928) records that they do not hibernate and are seen throughout the day during the winter, and only in early morning and late afternoon during the summer.
Koniecznyi's monitor feeds largely on invertebrates (especially beetles) lizards, reptile eggs, toads and small mammals. It finds most of its food below the ground or under debris. In the desert scrub of Pakistan densities are estimated at 9 specimens per km2. Mating occurs in .Iuly or August, during the monsoon and 2-15 eggs are laid in September and October (Auffenberg et al 1989). According to Auffenberg (1986) bipedal combat is characteristic of all Indian monitors and occurs in Koniecznyi's monitor between May and early July. Male Koniecznyi's monitors may become more intense in colour during the breeding season. Auffenberg et al (1989) suggest an incubation period of up to ten months. They also record a biased sex ratio of 2.23: 1 in favour of males.
Grey monitors of all races tend to do very poorly in captivity, rarely surviving for more than a few years. It seems to be essential that the animals are kept cool during the winter in order to stimulate their natural patterns of inactivity. Properly maintained these glorious reptiles have lived in excess of 17 years in captivity and if obtained as juveniles their lifespans could exceed 25 years (Bennett 1994b). Reliable reports of captive breeding occur only for V.griseus ,griseus (Perry et al 1993). Their animals were kept in hibernation at temperatures as low as 6°C from November to March. For the rest of the year two females and a male were kept in a 20m2 outside enclosure. Seven eggs, weighing about 25.5g each, were incubated at 29-31°C and hatched after 120 days into 10cm SVL (25cm TL) lizards. They were not fed immediately but kept in the dark at 15-200C for the next five months. After two years they had increased in size to 24cm SVL (59cm TL) and weighed 117-269g. In captivity they will accept a wide range of vertebrate and invertebrate prey. This species does not usually tolerate being handled and does not become docile, even after long periods in captivity.
There is some suggestion that the bite of the desert monitor really does have an adverse effect on people. Sopiev et al (1987) record that following a bite from a young Caspian monitor (in which the lizard seized a finger and chewed on it for a minute) the "victim" suffered dizziness, muscular aches and pains, accelerated hearbeat and had difficulty breathing through the mouth. After 24 hours however all symptoms had disappeared . According to Gorelov (1971) injections of saliva from Caspian monitors causes momentary paralysis when injected into small birds and rodents. This however is refuted by Auffenberg (1986) who claims that the salivary glands of desert monitors have no such properties. The desert monitor has been attributed with much more malevolent powers. According to Bogdanov (in Nicolskii 1915) if a desert monitor runs between a man's legs it can rob him of his sexual prowess and render him impotent. Its common name amongst the kirgizes, kasal, means illness and is derived from this tenifying superstition. In Algeria their ability to survive the bites of venomous snakes is supposed to be due to their habit of seeking Ollt and consuming plants which act as antidotes against the venom (Mammir, pers. comm.). In Turkey the desert monitor is known as zagar, or gomgomok. In Iraq it is urqhal. In Turkmenistan the Caspian monitor is known as zemzem and in Tadjikistan as ichke mere.
Attribution / Courtesy: Daniel Bennett. 1995. A Little Book of Monitor Lizards. Viper Press U.K.





