Soil Factors
Soil Acidity, Calcium, and Magnesium
Soil reaction or soil pH is an important factor affecting availability of boron in soils. Generally, boron becomes less available to plants with increasing soil pH. Several workers have observed negative correlations between plant boron accumulation and soil pH (67,183–185). In some studies in New Zealand, liming of the soil reduced boron concentration in the first cuts of alfalfa and red clover, particularly at higher rates of applied boron (123). Studies by Peterson and Newman (186) and Gupta and MacLeod (187) have shown that a negative relationship between soil pH and plant boron occurs when soil pH levels are greater than 6.3 to 6.5. The availability of boron to plants decreases sharply at higher pH levels, but the relationship between soil pH and plant boron at soil pH values below 6.5 does not show a definite trend.Liming of soil decreased the plant boron accumulation when soil boron reserves were high (188). They attributed this effect to a high calcium content. Beauchamp and Hussain (189) in their studies on rutabagas, found that increased calcium concentration in tissue generally increased the incidence of brown-heart. Wolf (185) found that magnesium had a greater effect on boron reduction in plants than did calcium, sodium, or potassium, but the differences between calcium and magnesium effects were small. However, no distinction was made between the effects of soil pH and levels of calcium or magnesium on boron accumulation.
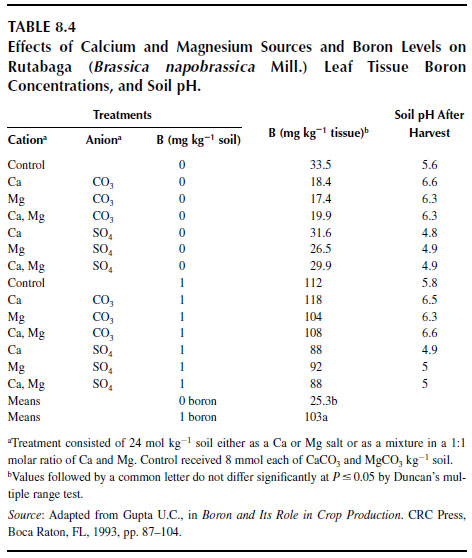
Experiments conducted to distinguish between the effects of soil pH and sources of calcium and magnesium showed that, in the absence of added boron, rutabaga roots and tops from calcium and magnesium carbonate treatments had more severe brown-heart condition than did roots from calcium and magnesium sulfate treatments (187). The leaf boron concentrations in rutabaga from treatments with no boron were lower at higher soil pH values where calcium or magnesium were applied as carbonates than they were at lower soil pH where sulfate was used as a source of calcium or magnesium (Table 8.4). In the presence of added boron, this trend was not clear, but the levels were well above the deficiency limit. The lower boron concentrations in the no-boron treatments with carbonates than in those with sulfates appear to be related to soil pH differences. These studies (187) showed no differences in boron accumulation whether the plants were fed with calcium or magnesium, as long as the corresponding anionic components were the same. Concentrations of calcium and magnesium, not shown in the table, were not related to the applications of boron. Table 8.4 shows that after the crop was harvested, lower quantities of hot-water-soluble boron were found in the soil that received calcium or magnesium sulfates than in soil that received calcium or magnesium carbonates.
 |
Unpublished data (83) on podzol soils with a pH range of 5.4 to 7.8 showed that liming markedly decreased the boron content of pea plant tissue from 117 to 198 mg kg-1 at pH 5.4 to 5.6, to 36 to 43 mg kg-1 at pH 7.3 to 7.5. At pH values higher than 7.3 to 7.5, even tripling the amount of lime did not affect the boron content of plant tissue.
No clear relationship was found between the Ca/B ratio in the leaf blades and the incidence of brown-heart in rutabaga (189). However, it was noted that an application of sodium increased the calcium concentration in rutabaga tissue, thereby affecting the Ca/B ratio and possibly the incidence of brown-heart. It should be pointed out that use of the Ca/B ratio in assessing the boron status of plants should be viewed in relation to the sufficiency of other nutrients in the growing medium and in the plant.
Macronutrients, Sulfur, and Zinc
boron, application of nitrogen depresses the level of boron in orange (Citrus sinensis Osbeck) leaves (192). Lysimeter experiments showed that tripled fertilization (NPK) rates and irrigation increased boron accumulation by plants on tested soils (193).Boron concentrations in boot-stage tissue of barley and wheat increased significantly with increasing rates of compost additions (59). Such increases in boron were attributed to a high concentration of 14 mg B kg-1 in the compost. The authors reported that boron concentrations decreased with increasing rates of nitrogen. Additions of nitrogen decreased the severity of boron toxicity symptoms. The form of nitrogen can affect plant boron accumulation. Wojcik (194) reported that on boron-deficient, coarse-textured soils, nitrogen as calcium and ammonium nitrates increased the availability and uptake of boron by roots. This increase was attributed to the fact that nitrate inhibited boron sorption on iron and aluminum oxides, and increased boron in soil solution.
Increasing rates of nitrogen applied to initially nitrogen-deficient soils significantly decreased the boron concentration of boot-stage tissue in barley and wheat in a greenhouse study, but field experiments did not show any significant effect of nitrogen on boron concentration (195). The ineffectiveness of nitrogen in alleviating boron toxicity in cereals under field conditions is due to the fact that nitrogen failed to decrease the boron concentration in boot-stage tissue. Furthermore, nitrogen deficiency was more severe under greenhouse conditions than under field conditions. The decreases in boron concentrations were greater with the first level of added nitrogen than with the higher rates (195). This result may indicate that application of nitrogen is helpful in alleviating boron toxicity on soils low in available nitrogen.
Little difference in boron concentration of alfalfa was detected, and symptoms of boron deficiency progressed with increasing potassium concentration in the growth media (196). The authors suggested that the accentuating effect of high potassium on boron toxicity or deficiency symptoms might be due to the influence of potassium on cell permeability, which is presumably regulated by boron. Long-term experiments on cotton indicated positive yield responses to boron fertilization when associated with potassium applications (197). Yield increases were related to increased leaf potassium and boron concentrations.
The effects of phosphorus, potassium, and sulfur are less clear than those of nitrogen on the availability of boron to plants. Studies conducted in China (198) showed that rape (Brassica napus L.) plant boron concentration decreased with increasing potassium, and that lower potassium levels enhanced boron accumulation. The authors concluded that the optimum K/B ratio in rape plants was 1000:1.
Tanaka (199) showed that boron accumulation in radish increased with an increase in phosphorus supply. Malewar et al. (200) found that increasing the phosphorus fertilization rate resulted in higher phosphorus in cotton and groundnut. Experiments conducted on cotton also demonstrated that boron concentration in leaves was greatest with phosphorus fertilization (201). On the other hand, the presence of phosphorus can affect boron toxicity in calcareous soils. In studies on maize genotypes, boron was more toxic in the absence, rather than in the presence of, phosphorus, and thus boron toxicity in calcareous soils of the semiarid regions could be alleviated with applications of phosphorus (202).
Sulfate may have a slight effect on accumulation of boron in plant tissues (199). Field studies in Maharashtra, India, showed that boron applied with gypsum gave increased dry pod yield of groundnuts (203). The experimental results from a number of crops indicated that sulfur applications had no effect on boron concentration of peas, cauliflower, timothy (Phleum pratense L.), red clover, and wheat, but such applications significantly decreased the boron content of alfalfa and rutabaga (83). It is possible that various crops behave differently. For example, with soybean, applications of gypsum at 1000 kg ha-1 did not alleviate boron toxicity resulting from the application of 10 kg B ha-1 (204).
Recent studies showed that applied zinc played a role in partially alleviating boron toxicity symptoms by decreasing the plant boron accumulation (205). Zinc treatments partially depressed the inhibitory effect of boron on tomato growth (150).
Soil Texture
The texture of soil is an important factor affecting the availability of boron (206). A study on soils from eastern Canada showed that higher quantities of hot-water-soluble boron occurred in finetextured soils than in coarse-textured soils (207). Studies in Poland showed that boron accumulation in potatoes and several cereals was less on sandy soils than on loamy soils (193).Page and Cooper (208) reported that leaching losses from acid, sandy soils after addition of 12.5 cm of water, account for as much as 85% of the applied boron. Movement is less rapid in heavy-textured soils because of increased fixation by the clay particles (119).In Brazil, response to boron by cotton was significantly higher on Alic Cambisol, and the reverse was true for a dystrophic dark red latosol (209). It was suggested that high sand content (87%) and low clay (10%) and low organic matter (1.3%) in the latter soil could have resulted in toxic concentrations of boron in solution. The type of clay and the soil pH can significantly influence the amount of boron adsorbed. Hingston (210) reported that increasing pH resulted in an increase in the monolayer adsorption and a decrease in bonding energy for Kent sand kaolinite and Marchagee montmorillonite, and a slight increase in bonding energy for Willalooka illite up to pH 8.5. On a mass basis, illite adsorbed most boron over the range of pH values commonly occurring in soils; montmorillonite adsorbed appreciable amounts at higher pH, and kaolinite adsorbed the least.
Fine-textured soils generally require more boron than do the coarse-textured soils to produce similar boron concentrations in plants. Boron concentrations in solutions of 3.5 mg kg-1 in sandy loam and 4.5 mg kg-1 in clay loam resulted in similar boron concentrations in gram (Cicer arietinum L.) (211).
Soil Organic Matter
Organic matter is one of the chief sources of boron in acid soils, as relatively little boron adsorption on the mineral fraction occurs at low pH levels (212). The hot-water-soluble boron in soil has been positively related to the organic matter content of the soil (207). Addition of materials such as compost rich in organic matter resulted in large concentrations of boron in plant tissues and in phytotoxicity (60). Boron in organic matter is released in available form largely through the action of microbes (213). The complex formation of boron with dihydroxy compounds in soil organic matter is considered to be an important mechanism for boron retention (214). The influence of organic matter on the availability of boron in soils is amplified by increases in pH and clay content of the soil.Soil Adsorption
When boron is released from soil minerals, mineralized from organic matter, or added to soils by means of irrigation or fertilization, part of the boron remains in solution, and part is adsorbed (fixed) by soil particles. An equilibrium exists between the solution and adsorbed boron (215). Usually more boron is adsorbed by soils than is present in solution at any one time (216), and fixation seems to increase with time (207).Boron retention in soil depends upon many factors such as the boron concentration of the soil, soil pH, texture, organic matter, cation exchange capacity, exchangeable ion composition, and the type of clay and mineral coatings on clays (210,215,217,218). Of the clays, illite is the most reactive with boron, and kaolinite is the least reactive on a mass basis (210,219).




