Osmotic Adjustments and Controlling Factors
Among the accumulators are amino acids, predominantly proline, sugars and
sugar alcohols, such as sucrose or mannitol, trehalose, mannitol/sorbitol, or
inositol derivatives, and more complex carbohydrates, such as fructans and
raffinose-related compounds. This list is likely to expand in the future as other
models are investigated. In a drought-adapted watermelon, for example, citrulline,
an intermediate in the urea cycle, has been detected as a drastically accumulating
metabolite during drought stress. Citrulline function may be in radical
oxygen scavenging (Akashi
et al., 2001, 2004). A number of experiments have
been reported that attempted to engineer osmolyte accumulation into glycophytic
plants that show only marginal accumulation of metabolites with the intention to
improve tolerance. Table 12.2 provides a selection of such studies, also including
experiments to transgenically engineer ionic stress tolerance and to engineer regulatory
circuits. The range included exemplifies the major categories of genes
or cDNAs that have been used in engineering: osmolytes and other protectants
[chaperones, late-embryogenesis-abundant (LEA) and heat-shock proteins (HSPs)],
transporters and pumps, scavengers of radicals, adjustments in hormone biosynthesis,
and regulatory genes, outlined in detail at: www.plantstress.com/Files%
5CAbiotic-stress_gene.htm. Under strictly controlled growth conditions, it has been
shown in many of these experiments that the plants exhibited showed increased
osmotic, ionic, or temperature tolerance (Table 12.2). Often the actual increase or
accumulation did not amount to concentrations found in the natural models gave
rise to the ‘‘compatible osmolyte’’ concept.
These experiments demonstrated several other aspects as well. First, osmotic/
ionic abiotic stress tolerance seems to
be controlled by different mechanisms
depending on age or developmental stage, that is, seedling, vegetative, and
reproductive stages, each seem to require stage-specific regulation of tolerance
and protective determinants (Rontein
et al., 2002).
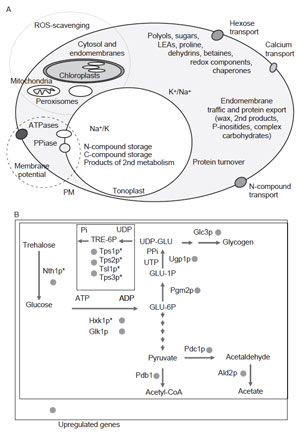 |
| FIGURE 12.2 Biochemical and metabolic
determinants of salinity stress tolerance.
(A) A schematic
representation of cellular
mechanisms depicted as functional categories
(e.g., maintenance
of membrane potential,
ROS-scavenging, altered membrane traffic, or
protein turnover), and
identification of major
metabolites and protein families that constitute
cellular defenses against
ionic stress
(modified after Hasegawa et al., 2000b).
Included are functions such as protein turnover,
membrane structure, and vesicular traffic
reorientations. Not included are molecular
functions that also play important roles: chromatin
remodeling, transcription/splicing, RNA
transport,
or regulation of translation. (B) Metabolic
reactions leading to trehalose synthesis and
reutilization in S. cerevisiae stressed by addition
of 1 M NaCl (90 min). Upregulated transcripts
are
indicated by filled circles, and the presence of
a stress response element (STRE) in promoters of
individual genes is indicated (*). Although
trehalose accumulates long term, the trehalose-
cleaving
enzyme, trehalase, is also upregulated
(Yale and Bohnert, 2001). |
In one of the first attempts at
engineering, for example, salinity stress tolerance (mannitol accumulation) was
observed only when the transgenic tobacco plants received stress during early
vegetative growth (Tarczynski
et al., 1993). Second, the common use of strong,
constitutively expressed regulatory elements, while potentially leading to high
(enzyme) expression, product accumulation, and vegetative tolerance, is or can be
nonphysiological. This was demonstrated by the high accumulation of D-ononitol
and mannitol in transgenic tobacco that protected the plants at vegetative growth
stages (salinity and drought), but prevented normal seed formation due to the
interference of the accumulating metabolites, both nonutilizable metabolic endproducts
in tobacco, with sucrose unloading in the developing seeds (Sheveleva
et al., 2000). Figure 12.2A gives a schematic rendition of biochemical and physiological
mechanisms, structures, and determinants that have recognized as stress
relevant from experiments conducted during the last decade.
Third, synthesis leading to accumulation is not necessarily the major purpose
of a purported osmolyte, while the accumulation may be a pathological side effect.
One example is yeast that increases the pathway leading to increased synthesis of
trehalose (Hohmann, 2002). However, yeast also increases levels of enzymes that
degrade trehalose, indicating that flux through the pathway (consuming reducing
power) may be more important than simply making more (Fig. 12.2B). This behavior has been shown in several studies (Yale and Bohnert, 2001). Only at
very high salt concentrations, which arrests or slows growth, will trehalose
accumulate drastically in yeast. Such a view might then lead to a different
interpretation for the compatible solutes. They might be seen as metabolic valves
that adjust or lower the redox potential of cells in order to prevent or minimize
production of radical oxygen species (ROS) in mitochondria and chloroplasts.
Finally, attempts at engineering salinity stress tolerance have only begun to pay
attention to compartment-specific strategies and the appreciation of stress tolerance
as a multigenic network has not been tackled, for example, by the accumulation
of different stress alleviating determinants.